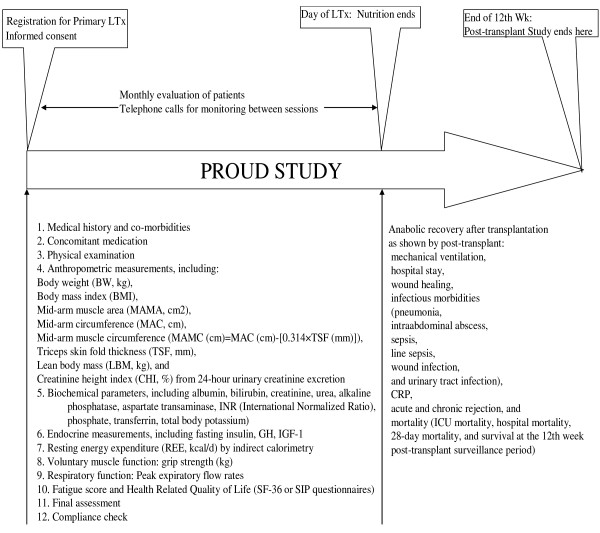
Figure 1.
Scheme depicting the workflow of the study. Each subject will be recruited on the day of registration on the waiting list for liver transplantation after giving informed consent. Study nutrition ends on the day of transplantation. Subjects are randomized to two parallel groups treated for study or control product. Furthermore, patients will be followed for 12 weeks after transplantation.