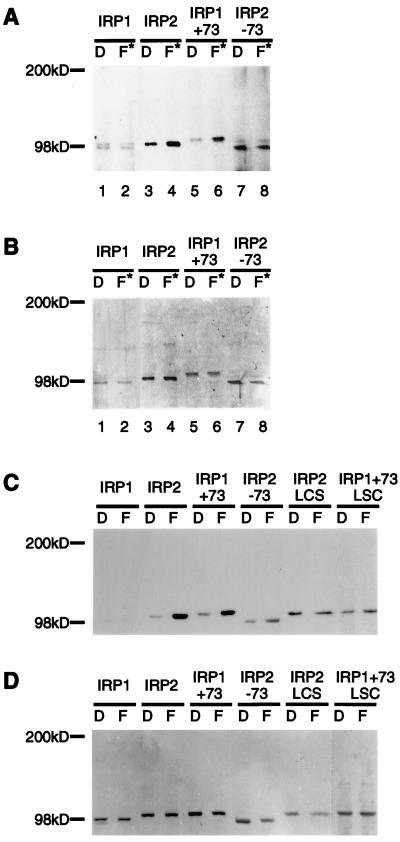
Figure 2.
IRP2 and related proteins are subject to iron-dependent oxidation in vivo. RD4 stable transformants expressing IRP1, IRP2, IRP1+73, and IRP2–73 were cultured for 24 h in the presence of dexamethasone (20 nM) to induce protein expression, then were treated with either Df (D) or FAC plus lactacystin (10 μM) (F*) for 12 h in the continuous presence of dexamethasone. IRPs in cell extracts were quantitated by Western blotting with anti-myc antibody, and equal amounts of recombinant protein from each treatment were immunoprecipitated from the lysates and derivatized by DNP as described in Materials and Methods. Proteins separated on an 8–16% SDS/PAGE were transferred, and carbonyl groups were detected with anti-DNP antibodies (A). Membranes then were stained with Amido Black (Sigma) (B). COS cells transiently transfected with IRP constructs were cultured in the presence of 100 μM Df (D) or 400 μg/ml FAC (F) for 24 h. The amounts of recombinant IRP in cell extracts were determined by Western blot analysis with anti-myc antibody by using purified IRP2 protein as a standard. Immunoprecipitated recombinant proteins (0.5 pmol) were derivatized with DNP, and carbonyl modifications were detected by Western blot analysis with anti-DNP antibody (C). To verify that equivalent amounts of proteins were assayed, membranes then were stained with Coomassie brilliant blue (D).