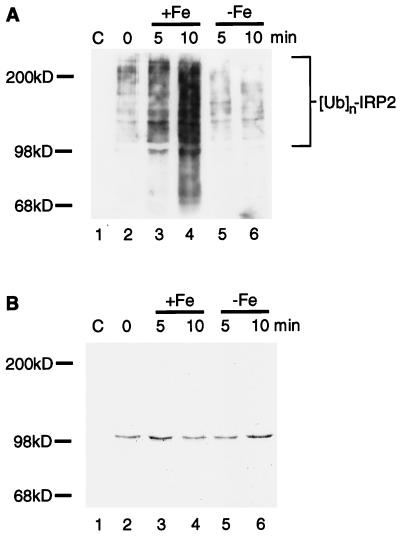
Figure 4.
Iron-catalyzed oxidation of IRP2 creates a substrate for in vitro ubiquitination. (A) Purified IRP2 (lanes 2–6) was incubated with 10 mM DTT in the presence or absence of 5 μM FeCl3 at 37°C for 5 min (lanes 3 and 5, respectively) or 10 min (lanes 4 and 6, respectively). Reactions were stopped by addition of 100 μM Df. RD4 cell lysates (400 μg) supplemented with ATP (2 mM) and ubiquitin (6 μg) (Sigma) were added to tubes containing no IRP2 (lane 1), native IRP2 (lane 2), in vitro-oxidized IRP2 (lanes 3 and 4), or IRP2 treated with DTT alone (lanes 5 and 6). Anti-myc immunoprecipitates of each lane were resolved by 6% SDS/PAGE, and Western blotting with antiubiquitin antiserum (A) was followed by staining of the same membrane with Coomassie brilliant blue (B).