Abstract
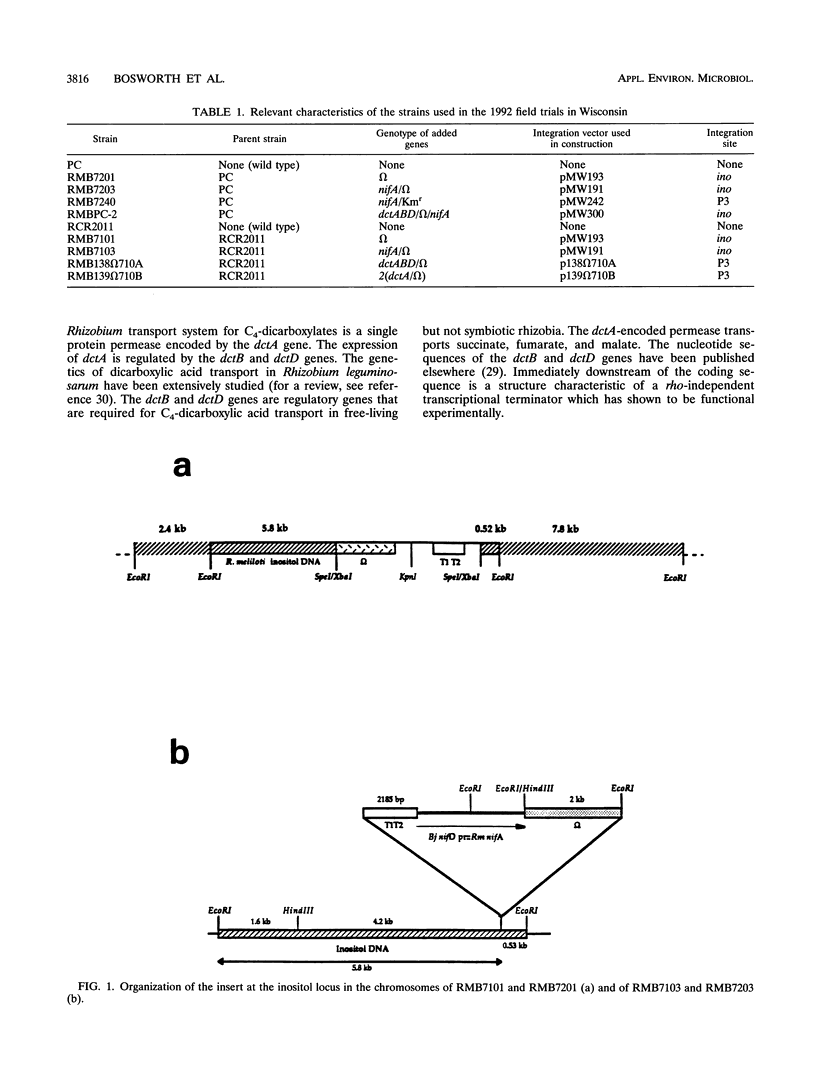
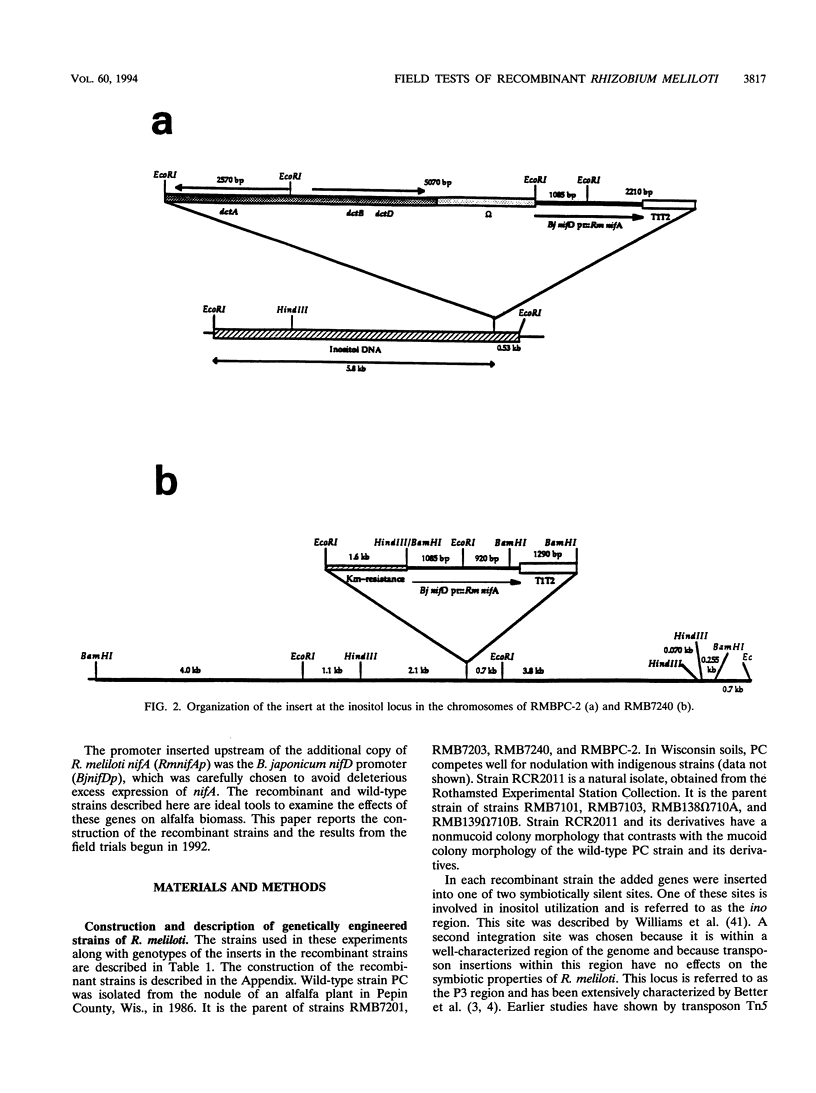
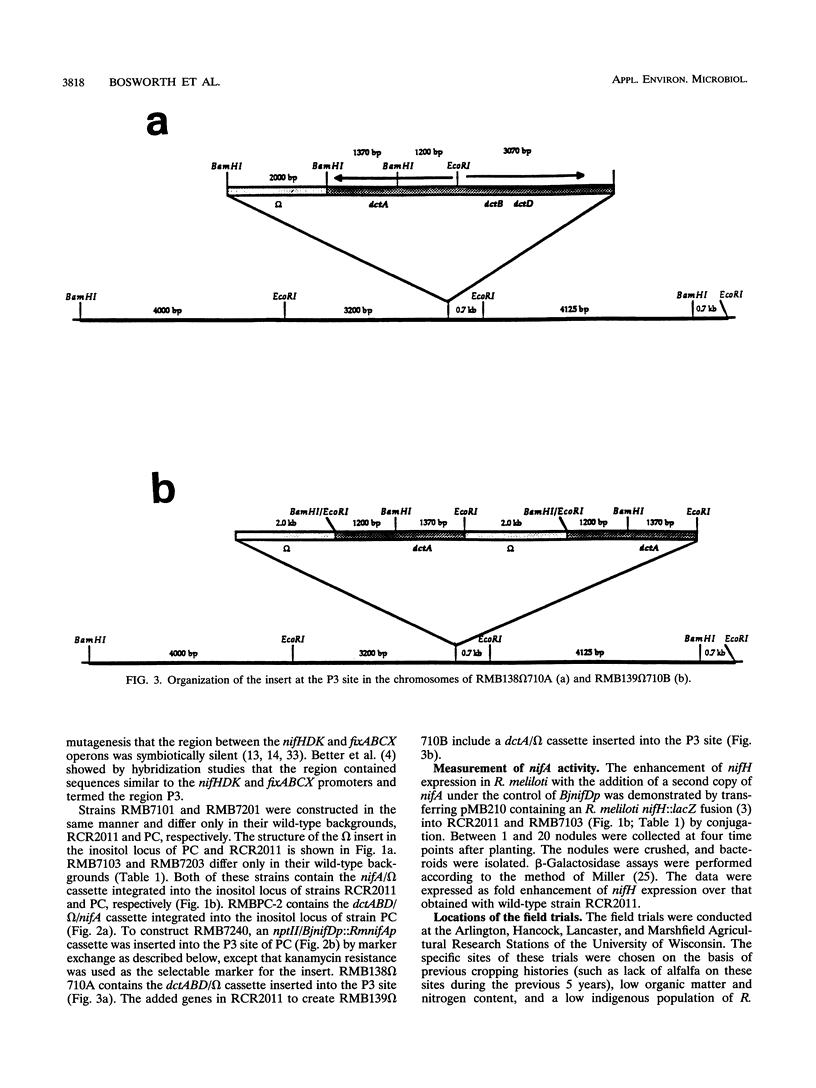
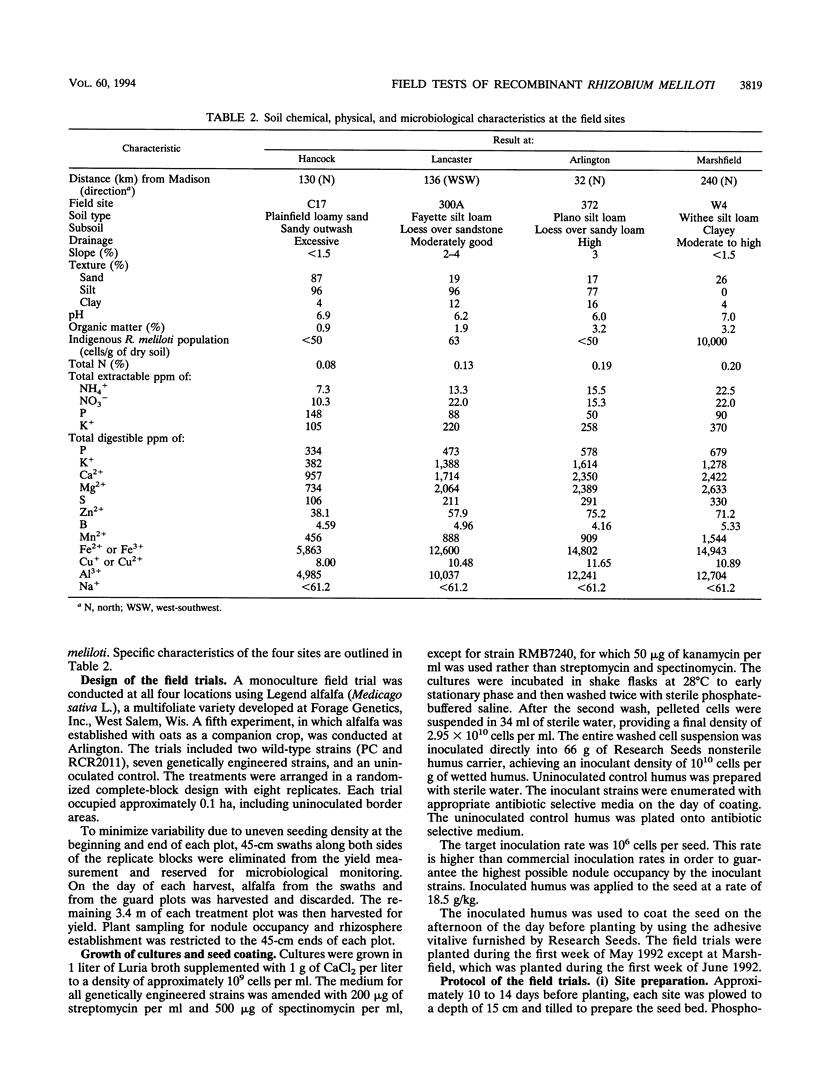
The construction of rhizobial strains which increase plant biomass under controlled conditions has been previously reported. However, there is no evidence that these newly constructed strains increase legume yield under agricultural conditions. This work tested the hypothesis that carefully manipulating expression of additional copies of nifA and dctABD in strains of Rhizobium meliloti would increase alfalfa yield in the field. The rationale for this hypothesis is based on the positive regulatory role that nifA plays in the expression of the nif regulon and the fact that a supply of dicarboxylic acids from the plant is required as a carbon and energy source for nitrogen fixation by the Rhizobium bacteroids in the nodule. These recombinant strains, as well as the wild-type strains from which they were derived, are ideal tools to examine the effects of modifying or increasing the expression of these genes on alfalfa biomass. The experimental design comprised seven recombinant strains, two wild-type strains, and an uninoculated control. Each treatment was replicated eight times and was conducted at four field sites in Wisconsin. Recombinant strain RMBPC-2, which has an additional copy of both nifA and dctABD, increased alfalfa biomass by 12.9% compared with the yield with the wild-type strain RMBPC and 17.9% over that in the uninoculated control plot at the site where soil nitrogen and organic matter content was lowest. These increases were statistically significant at the 5% confidence interval for each of the three harvests made during the growing season. Strain RMBPC-2 did increase alfalfa biomass at the Hancock site; however, no other significant increases or decreases in alfalfa biomass were observed with the seven other recombinant strains at that site. At three sites where this experiment was conducted, either native rhizobial populations or soil nitrogen concentrations were high. At these sites, none of the recombinant strains affected yield. We conclude that RMBPC -2 can increase alfalfa yields under field conditions of nitrogen limitation, low endogenous rhizobial competitors, and sufficient moisture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. H., McClung C. R., Chelm B. K. Physical organization of the Bradyrhizobium japonicum nitrogenase gene region. J Bacteriol. 1984 Sep;159(3):857–862. doi: 10.1128/jb.159.3.857-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Ditta G., Helinski D. R. Deletion analysis of Rhizobium meliloti symbiotic promoters. EMBO J. 1985 Oct;4(10):2419–2424. doi: 10.1002/j.1460-2075.1985.tb03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Birkenhead K., Manian S. S., O'Gara F. Dicarboxylic acid transport in Bradyrhizobium japonicum: use of Rhizobium meliloti dct gene(s) to enhance nitrogen fixation. J Bacteriol. 1988 Jan;170(1):184–189. doi: 10.1128/jb.170.1.184-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Long S. R., Brown S. E., van den Bos R. C., Earl C., Ausubel F. M. Physical and genetic characterization of Rhizobium meliloti symbiotic mutants. J Mol Appl Genet. 1983;2(3):249–260. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Richardson A. E., Gartner E., Djordjevic M. A., Roughley R. J., Rolfe B. G. Construction of an Acid-Tolerant Rhizobium leguminosarum Biovar Trifolii Strain with Enhanced Capacity for Nitrogen Fixation. Appl Environ Microbiol. 1991 Jul;57(7):2005–2011. doi: 10.1128/aem.57.7.2005-2011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D., Ditta G., Helinski D. R. Clustering of nitrogen fixation (nif) genes in Rhizobium meliloti. J Bacteriol. 1982 Jan;149(1):221–228. doi: 10.1128/jb.149.1.221-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Harker A. R., Papen H., Russell S. A., Hanus F. J., Zuber M. Physiology, biochemistry, and genetics of the uptake hydrogenase in rhizobia. Annu Rev Microbiol. 1987;41:335–361. doi: 10.1146/annurev.mi.41.100187.002003. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. R., Beringer J. E. A physical map of pPH1JI and pJB4JI. Plasmid. 1984 Sep;12(2):139–141. doi: 10.1016/0147-619x(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Jiang J., Gu B. H., Albright L. M., Nixon B. T. Conservation between coding and regulatory elements of Rhizobium meliloti and Rhizobium leguminosarum dct genes. J Bacteriol. 1989 Oct;171(10):5244–5253. doi: 10.1128/jb.171.10.5244-5253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Mutant Strains of Rhizobium japonicum with Increased Ability to Fix Nitrogen for Soybean. Science. 1978 Aug 4;201(4354):448–450. doi: 10.1126/science.201.4354.448. [DOI] [PubMed] [Google Scholar]
- Paau A. S. Improvement of Rhizobium inoculants. Appl Environ Microbiol. 1989 Apr;55(4):862–865. doi: 10.1128/aem.55.4.862-865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Astwood P. M., Downie J. A. Molecular cloning and genetic organization of C4-dicarboxylate transport genes from Rhizobium leguminosarum. J Bacteriol. 1984 Dec;160(3):903–909. doi: 10.1128/jb.160.3.903-909.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Astwood P. M., Nixon B. T., Ausubel F. M. Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucleic Acids Res. 1987 Oct 12;15(19):7921–7934. doi: 10.1093/nar/15.19.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Scott D. B., Hennecke H., Lim S. T. The biosynthesis of nitrogenase MoFe protein polypeptides in free-living cultures of Rhizobium japonicum. Biochim Biophys Acta. 1979 Dec 17;565(2):365–378. doi: 10.1016/0005-2787(79)90212-0. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Okker R. J., Wijffelman C. A., Tak T., Goosen-de Roo L., Pees E., van Brussel A. A., Lugtenberg B. J. Symbiotic properties of rhizobia containing a flavonoid-independent hybrid nodD product. J Bacteriol. 1989 Jul;171(7):4045–4053. doi: 10.1128/jb.171.7.4045-4053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


