Abstract
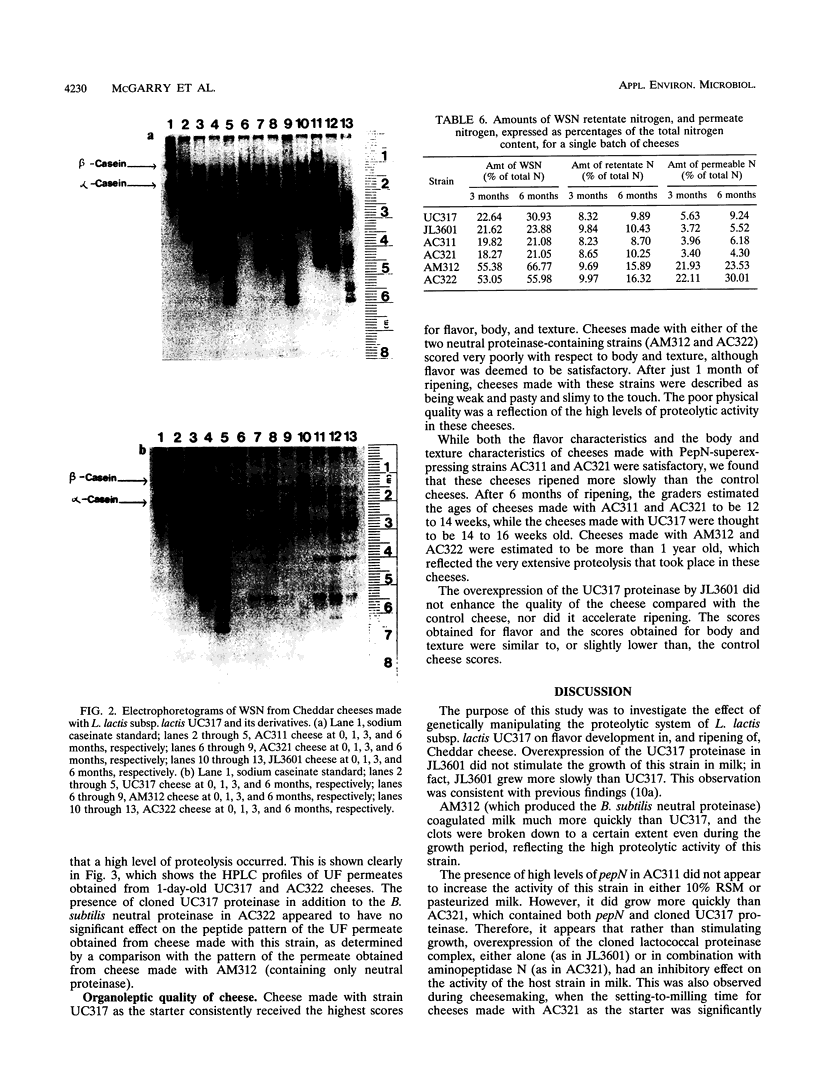
Three batches of six Cheddar cheeses were manufactured by using the following lactococcal strains: (i) UC317 as a control; (ii) JL3601, a proteinase-negative derivative of UC317 transformed with high-copy-number plasmid pCI3601 containing the cloned proteinase gene complex from UC317; (iii) AM312, a proteinase-negative derivative of UC317 transformed with plasmid pMG36enpr containing the neutral proteinase gene from Bacillus subtilis; (iv) AC322, JL3601 transformed with pMG36enpr; (v) AC311, UC317 transformed with plasmid pNZ1120, which contains the aminopeptidase N (pepN) gene from Lactococcus lactis subsp. lactis MG1363; and (vi) AC321, JL3601 transformed with pNZ1120. Organoleptic and chemical analyses indicated that (i) the control cheeses, which were made with UC317, were of the highest quality; (ii) cheeses made with strains harboring pCI3601 in addition to either pMG36enpr (AC322) or pNZ1120 (AC321) did not ripen in a significantly different manner than cheeses made with AM312 (containing only pMG36enpr) or AC311 (containing only pNZ1120), respectively; (iii) cheeses made with strains that overproduce pepN did not have improved body, texture, and flavor characteristics; and (iv) cheeses made with strains harboring the neutral proteinase from B. subtilis (AM312 and AC322) underwent greatly accelerated proteolysis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews A. T. Proteinases in normal bovine milk and their action on caseins. J Dairy Res. 1983 Feb;50(1):45–55. doi: 10.1017/s0022029900032519. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Boezi J. A. A new staining technique for proteins in polyacrylamide gels using coomassie brilliant blue G250. Anal Biochem. 1977 Oct;82(2):580–582. doi: 10.1016/0003-2697(77)90197-x. [DOI] [PubMed] [Google Scholar]
- Coffey A. G., Fitzgerald G. F., Daly C. Cloning and characterization of the determinant for abortive infection of bacteriophage from lactococcal plasmid pCI829. J Gen Microbiol. 1991 Jun;137(6):1355–1362. doi: 10.1099/00221287-137-6-1355. [DOI] [PubMed] [Google Scholar]
- Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Law J., Fitzgerald G. F., Uniacke-Lowe T., Daly C., Fox P. F. The contribution of lactococcal starter proteinases to proteolysis in cheddar cheese. J Dairy Sci. 1993 Sep;76(9):2455–2467. doi: 10.3168/jds.S0022-0302(93)77580-3. [DOI] [PubMed] [Google Scholar]
- Law J., Vos P., Hayes F., Daly C., de Vos W. M., Fitzgerald G. Cloning and partial sequencing of the proteinase gene complex from Lactococcus lactis subsp. lactis UC317. J Gen Microbiol. 1992 Apr;138(4):709–718. doi: 10.1099/00221287-138-4-709. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alen-Boerrigter I. J., Baankreis R., de Vos W. M. Characterization and overexpression of the Lactococcus lactis pepN gene and localization of its product, aminopeptidase N. Appl Environ Microbiol. 1991 Sep;57(9):2555–2561. doi: 10.1128/aem.57.9.2555-2561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Guchte M., Kodde J., van der Vossen J. M., Kok J., Venema G. Heterologous gene expression in Lactococcus lactis subsp. lactis: synthesis, secretion, and processing of the Bacillus subtilis neutral protease. Appl Environ Microbiol. 1990 Sep;56(9):2606–2611. doi: 10.1128/aem.56.9.2606-2611.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]