Abstract
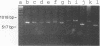
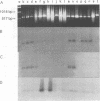
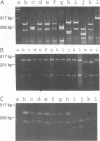
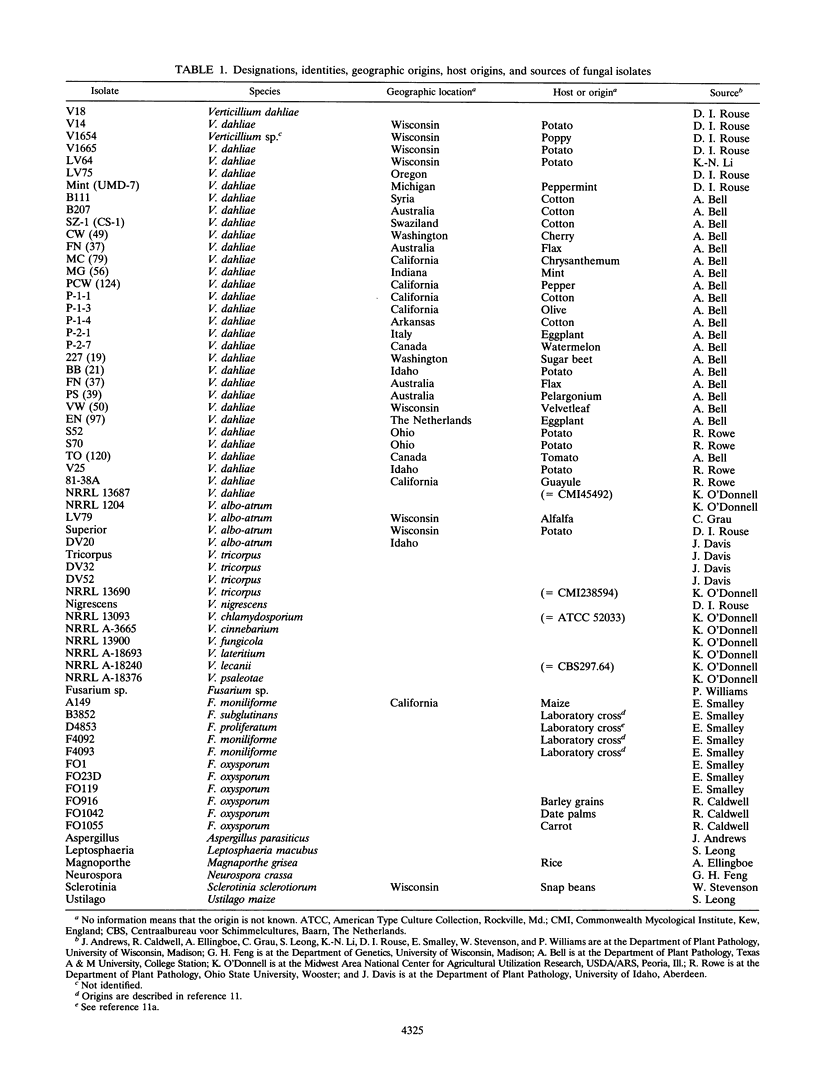
A pair of conserved PCR primers, designated NMS1 and NMS2, that amplify a region in the mitochondrial small rRNA gene region were designed for fungi belonging to the class Ascomycetes. These primers were tested with members of eight fungal genera (Aspergillus, Fusarium, Magnaporthe, Mycospharella, Neurospora, Saccharomyces, Sclerotinia, Verticillium) and 10 Verticillium species (Verticillium albo-atrum, Verticillium chlamydosporium, Verticillium cinnebarium, Verticillium dahliae, Verticillium fungicola, Verticillium lecanii, Verticillium lateritium, Verticillium nigrescens, Verticillium psaliotae, and Verticillium tricorpus). The primers were also tested with 35 isolates of V. dahliae obtained from diverse geographic areas and diverse hosts. The results of a restriction fragment length polymorphism analysis of the region amplified by the primers differentiated the genera examined and the results of a DNA sequence analysis of the amplified region differentiated the Verticillium species. Two Fusarium species were also differentiated by the results of the restriction fragment length polymorphism analysis. On the basis of the nucleotide sequences of the amplified regions, we obtained a pair of PCR primers that could be used to differentiate V. dahliae from the other fungal isolates tested, including V. albo-atrum, a closely related plant-pathogenic species. The V. dahliae-specific PCR primer may aid in more rapid and specific detection of the pathogen directly in plant and/or soil samples. PCR primers NMS1 and NMS2 may be used as potential mitochondrial markers for studying fungal cytoplasmic inheritance of ascomycetes and for identifying DNA probes that are informative at or below the genus level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R., Olsen G. J., Woese C. R. Ribosomal RNA phylogeny and the primary lines of evolutionary descent. Cell. 1986 May 9;45(3):325–326. doi: 10.1016/0092-8674(86)90315-6. [DOI] [PubMed] [Google Scholar]
- Persing D. H. Diagnostic molecular microbiology. Current challenges and future directions. Diagn Microbiol Infect Dis. 1993 Feb;16(2):159–163. doi: 10.1016/0732-8893(93)90015-y. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]