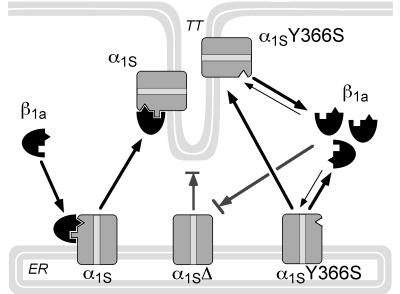
Figure 6.
Model of α1S–β1a interactions in the assembly of the DHP receptor complex in the skeletal muscle triad. β1a without the α1S subunit is localized in the cytoplasm. On coexpression β1a and α1S form a complex in the endoplasmic reticulum that is then incorporated into the junctional T-tubules (TT). Deletion of the β interaction domain in the I–II cytoplasmic loop blocks β1a association and export of the α1S mutant (α1S-Δ) from the endoplasmic reticulum. A point mutation (α1S-Y366S) in the high affinity β1a binding site of α1S (symbolized by the rectangular nipple) inhibits α1S/β1a complex formation but not the functional incorporation of the mutated α1S-Y366S into the T-tubules. Dynamic interactions between free cytoplasmic β1a and a low affinity interaction site (symbolized by the triangular notch) may modulate Ca2+ currents of α1S-Y366S and may be involved in the translocation of α1S-Y366S into the T-tubules.