Abstract
Background
Surgical resection remains the gold standard in dealing with liver tumours. Blood loss, biliary leak and postoperative liver function are still the main concerns of surgeons operating on the liver, even though different techniques have been developed to allow safer liver resection. A novel concept for liver resection is described using a radiofrequency energy (RF) assisted technique.
Method
A patient with a large colorectal liver metastasis located in segments VI, VII, VIII underwent a right hepatectomy using this technique. At laparotomy the tumour was staged with intraoperative ultrasonography, and a ‘cooled tipped’ radiofrequency probe was used to achieve a ‘zone of desiccation’ in the liver parenchyma 2 cm away from the edge of the tumour. Liver parenchyma was subsequently divided with a surgical scalpel.
Results
The resection time was 80 min with a blood loss of 30 ml. The patient was discharged on the ninth postoperative day without complications.
Discussion
Liver resection assisted by RF energy is feasible and safe. This technique could offer a new method for ‘transfusion-free’ resection without the need for sutures, ties, staples, tissue glue or admission to the intensive care unit.
Keywords: liver resection, radiofrequency ablation, liver tumours
Introduction
Operative treatment is the best method of dealing with liver tumours. Although the results of liver resection have improved over the last decade, blood loss, biliary leak and postoperative liver function are still major postoperative complications that cause concern 1,2,3,4,5,6. Radiofrequency (RF) energy has increasingly been used to ablate locally unresectable hepatic disease 7,8,9. Ionic agitation from an alternating current causes tissue coagulative desiccation through frictional heating. To maximise the potential benefit of alternating current energy application, a new technique for liver resection using RF energy has been developed by the senior author (NAH). RF energy is used to obtain coagulative desiccation of the resection margins, which can then be divided with a surgical scalpel.
Operative Technique
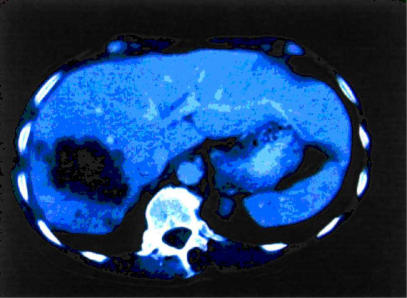
A 79-year-old woman with a solitary colorectal liver metastasis underwent a right hepatectomy following careful pre-operative staging with spiral computed tomography (CT) scan of chest and abdomen (Figure 1). The indication for operation was discussed at the Multi Disciplinary Treatment Pathway Meeting (MDTP), and written consent was obtained from the patient.
Figure 1. .
Preoperative CT scan showing colorectal metastasis in the right liver
Under general anaesthesia, a modified right subcostal incision was performed. The peritoneal cavity was examined for evidence of extrahepatic disease, and intraoperative ultrasonography (IOUS) was performed to exclude previously undetected lesions. The liver was then mobilised. Pringle's manoeuvre was not required. Landmarks were drawn on the surface of the liver before proceeding with the resection. Using argon diathermy, a line was made on the liver capsule to mark the periphery of the tumour assisted by bi-manual palpation and IOUS.
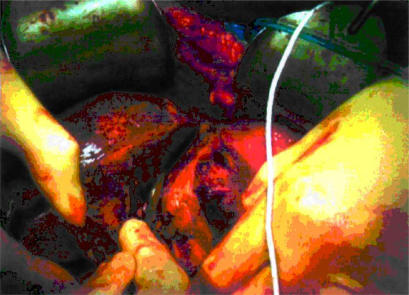
Coagulative desiccation was performed 2 cm away from the edge of the tumour using the ‘cooled-tip’ RF probe and a 500 kHz RF Generator (model RFG-3D-Radionics Europe, N.V., Wettdren, Belgium), which produces 100W of power and allows measurement of the generator output, tissue impedance and electrode tip temperature. The probe contains a 3-cm exposed electrode, a thermocouple on the tip to monitor temperature and impedance, and two coaxial cannulae through which chilled saline is circulated during RF energy application to prevent tissue boiling and cavitation immediately adjacent to the needle. Each probe application creates a ‘zone of desiccation’ in a core of tissue measuring 1 cm in radius and 3 cm in depth. Thus the number of probe applications required to obtain a ‘zone of desiccation’ is related to the thickness of the proposed cut surface of the liver to achieve resection. Application of the RF energy began with the area deepest and farthest from the upper surface of the liver. Once the deepest 3 cm of tissue was coagulated, the probe was withdrawn for a distance of 3 cm to coagulate the next vertical cylinder of tissue and so on until the upper surface of the liver was reached. Once an area was coagulated, the probe was withdrawn completely and placed 1–2 cm away from the previous application. After a ‘zone of desiccation’ in the liver parenchyma (2 cm away from the edge of the tumour) was completed, the liver parenchyma was divided with a surgical scalpel (Figures 2 and 3).
Figure 2. .
After radiofrequency energy application the liver parenchyma is transected with a scalpe
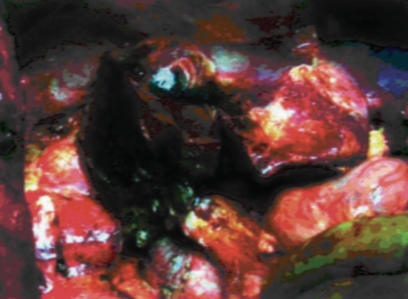
Figure 3. .
View of the operative field at the end of resection
After resection the ‘cool tip’ probe was inserted into the cut surface to stop any persistent bleeding points and to increase the safety resection margin as required. A drain was placed at the site of resection, and the abdomen was closed in layers.The procedure was completed as planned without the need for sutures, ties, staples or tissue glue. The resection time was 80 minutes, and blood loss during division of the liver parenchyma was 30 ml. The preoperative and postoperative haemoglobin values were 12.1 g/dL and 10.9 g/dL respectively. The patient did not require postoperative intensive care unit (ICU) admission or blood transfusion and was discharged on the ninth postoperative day without complications.
Discussion
Excessive intraoperative bleeding is associated with an increased incidence of postoperative complications and shorter long-term survival. Different techniques have been developed over the years to reduce blood loss during the transection phase 1,2,3,4,5,6. Surgeons can use low central venous pressure anaesthesia, continuous or intermittent hepatic pedicle clamping, or total vascular exclusion. Parenchymal division can be performed with the scalpel, by crushing with fingers or clamps, using ultrasonic dissectors and hydrodissectors or stapling devices. However, late bleeding and bile leak are possible even when ‘hi-tech’ devices are used because of insufficient tying of vessels or oozing from the resection surface secondary to tearing of small vessels.
The authors report an innovative technique which uses RF energy to assist liver resection and does away with the need for sutures, surgical knots, clips or glue. Radiofre-quency energy has increasingly been used for local ablation of unresectable hepatic disease 7,8,9. During ablation, ionic agitation from an alternating current causes tissue coagulative desiccation through frictional heating. The innovative step with this technique is that RF energy is applied to normal liver tissue surrounding the lesion to seal vascular and biliary structures along the plane of the intended resection. By contrast to coagulation of liver tumour tissue, coagulation of normal liver parenchyma is very rapid; only 40–60 seconds are required to create a ‘zone of desiccation’ in a core of tissue measuring 1 cm in radius and 3 cm in depth. In this case it took 80 minutes to perform the right hepatectomy.
In conclusion, the technique allowed a major liver resection to be performed with near-zero blood loss, without inflow vascular occlusion and without postoperative admission to the ICU or blood transfusion. The main advantage of the technique is that it is easy to teach and surgeons with a good knowledge of liver anatomy can apply it to both segmental and major resections.
There are obvious limitations to the technique. The first is that RF energy cannot be applied too close to the hilus or the vena cava because of fear of damaging these structures. The second is that it sacrifices parenchymal tissue that is usually spared using other resectional techniques.
Although further improvement of the RF probe and generator are needed, the technique described is feasible, safe and has the possibility to simplify hepatic parenchymal haemostasis and transection and allow a wider diffusion of liver surgery as it is simple to teach, to learn and to master.
Editor's note
This is the second of an occasional series of articles on operative technique or other interventional procedures in HPB medicine and surgery. Readers are invited to submit similar technical notes using the same format. The technical description should be succinct yet sufficiently detailed to be fully understandable. A few key references are desirable together with illustrative photographs or diagrams.
Robin Williamson
References
- 1.Bismuth H. Major hepatic resection under total vascular exclusion. Ann Surg. 1989;210:13–19. doi: 10.1097/00000658-198907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brancatisano R, Isla A, Habib N. Is radical hepatic surgery safe? Am J Surg. 1998;175:161–3. doi: 10.1016/S0002-9610(97)00265-1. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectalmetastases. J Clin Oncol. 1997;15:938–46. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 4.Hansen PD, Isla AM, Habib NA. Liver resection using total vascular exclusion, scalpel division of the parenchyma and a simple compression technique for haemostasis and biliary control. J Gastrointest Surg. 1999;3:537–42. doi: 10.1016/s1091-255x(99)80109-7. [DOI] [PubMed] [Google Scholar]
- 5.Nuzzo G, Guiliante F, Giovianni I, et al. Hepatic resections in normothermic ischaemia. Surgery. 1996;120:852–8. doi: 10.1016/s0039-6060(96)80094-8. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Ikai I, Kume M, et al. New technique for hepatic parenchymal resection using a cavitron ultrasonic surgical aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg. 1999;23:1032–7. doi: 10.1007/s002689900619. [DOI] [PubMed] [Google Scholar]
- 7.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiao LR, Hansen PD, Havlik R, et al. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999;177:303–6. doi: 10.1016/s0002-9610(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 9.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–46. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]