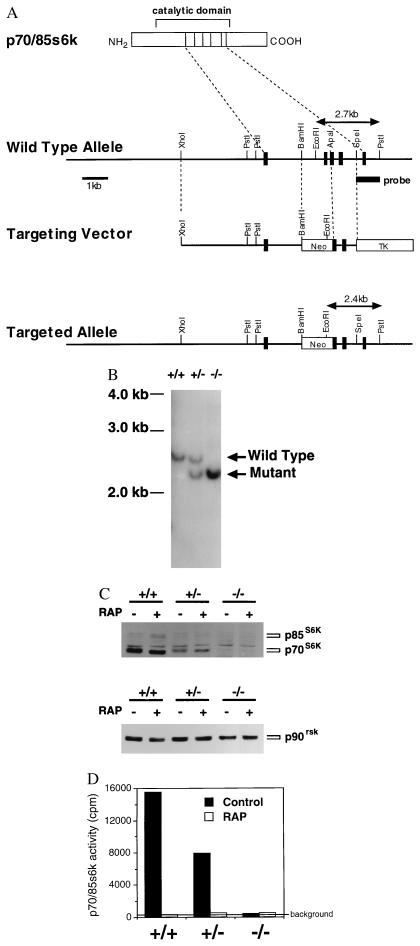
Figure 1.
Targeted disruption of the p70s6k gene in mouse ES cells. (A) Structure of the wild-type allele, targeting vector, and targeted allele. A portion (14 kb) of the p70/85s6k gene was cloned from the 129SV mouse genomic library. The neo selection cassette was inserted in the opposite orientation at BamHI and ApaI sites to disrupt an exon encoding a part of the catalytic domain (corresponding amino acids 207–237 in the p70s6k protein). The downstream coding region is designed to be frame-shifted. PGKtk cassette was then cloned at the 3′ SpeI site. The resulting targeting vector featured 5 kb of homologous sequence on the long arm and 1.2 kb on the short arm. (B) Southern blotting. The R1 ES cell line was transfected with the targeting vector. Colonies were picked after 12 days in selection medium and expanded. For isolating cells homozygous for the targeted allele, a heterozygous clone was grown on feeder cells in 6 mg/ml G418. Resistant colonies were picked after 10 days in high-concentration selection medium. Genomic DNA was extracted from cultured cells, digested with PstI and EcoRI, and separated in 1% agarose gel. After denature and neutralization of the gel, DNA was transferred to nylon membrane and hybridized with the 1.0-kb probe indicated in A. (C) Western blotting. Cells were treated with either vehicle (0.1% ethanol, Control) or rapamycin (10 ng/ml, RAP) for 30 min. Proteins were extracted from cells, separated on SDS/8% polyacrylamide gels, and transferred to nitrocellulose membranes. Immunoblotting was performed by using the ECL method. Antibodies used here were: for p70s6k and p85s6k, a rabbit polyclonal antibody raised against the common carboxyl-terminal sequence (C18); for p90rsk, a rabbit polyclonal antibody raised against the carboxyl terminus of the kinase. (D) Kinase activity. Cells were prepared as described above. The specific activities of p70s6k and p85s6k were measured by using the C18 antibody and S6 peptide as a substrate.