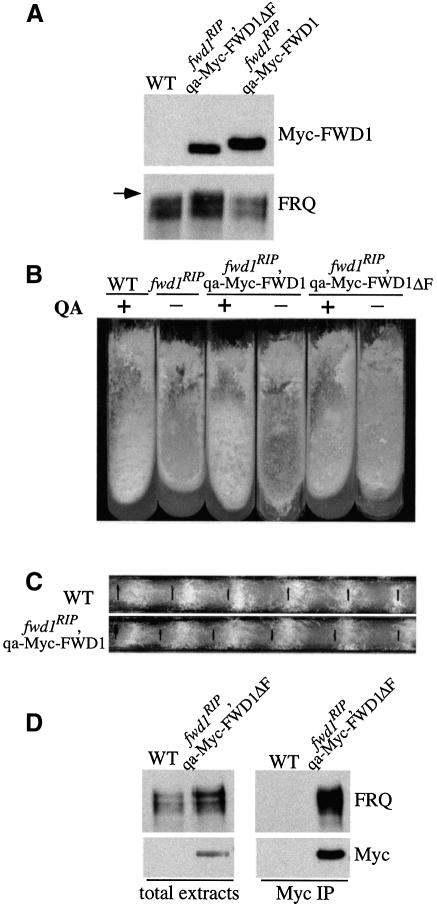
Fig. 5. FRQ and FWD1 interact physically in vivo. (A) Western blot analyses of the expression of FRQ and the Myc-FWD1 or Myc-FWD1ΔF proteins in fwd1RIP strains. QA was added into all cultures grown in LL. Note that the FRQ expression profile in the fwd1RIP,qa-Myc-FWD1 strain was similar to that of the wild-type strain (WT), while in the fwd1RIP,qa-Myc-FWD1ΔF strain, the FRQ profile was similar to that of the fwd1RIP mutants. The arrow indicates the hyperphosphorylated FRQ species observed in the fwd1RIP,qa-Myc-FWD1ΔF strain. (B) Growth phenotype of different strains in slants showing the rescue of the fwd1RIP phenotypes by the expression of the Myc-FWD1 protein, but not Myc-FWD1ΔF. (C) Circadian conidiation rhythm in the fwd1RIP,qa-Myc-FWD1 strain in the presence of QA (10–3 M). (D) Immunoprecipitation assay showing that FRQ and Myc-FWD1ΔF form a complex in vivo. The wild-type strain (WT) was used as the negative control. Cultures were grown in LL in the presence of QA (10–2 M). The total lysates or the immunoprecipitates were analyzed by immunoblotting using FRQ or c-Myc antibodies. The result shown is a representative example of multiple independent experiments.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.