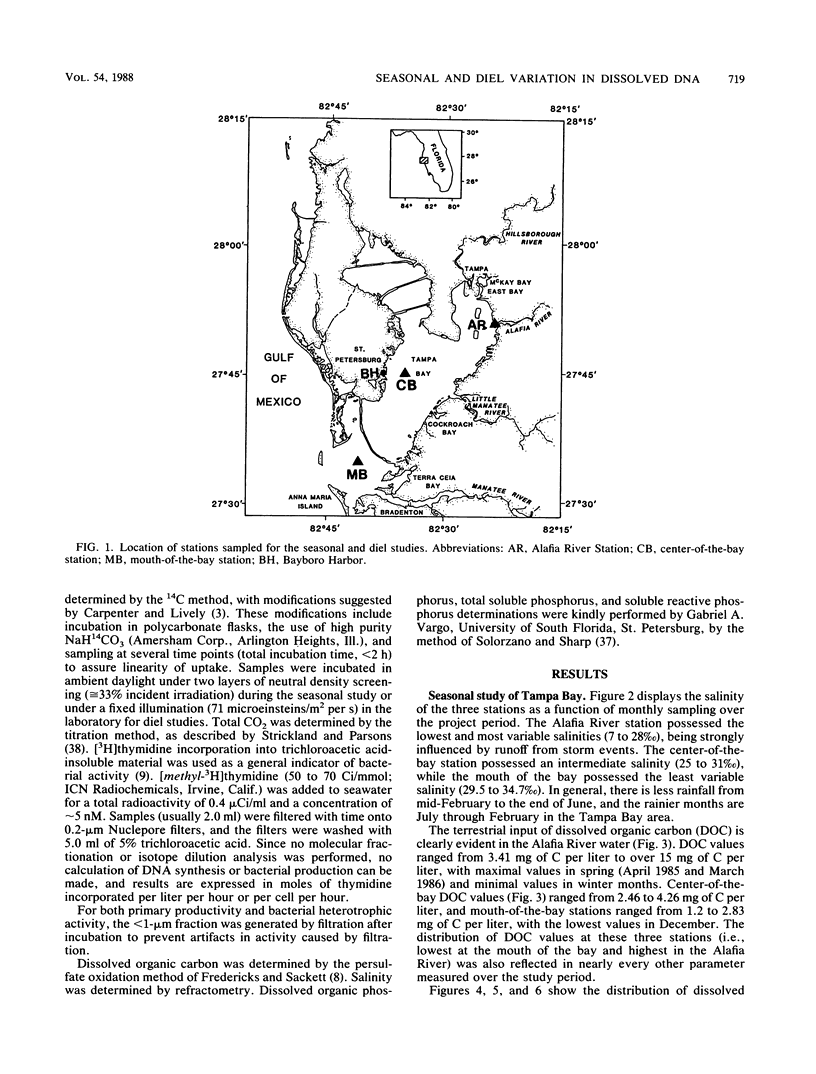
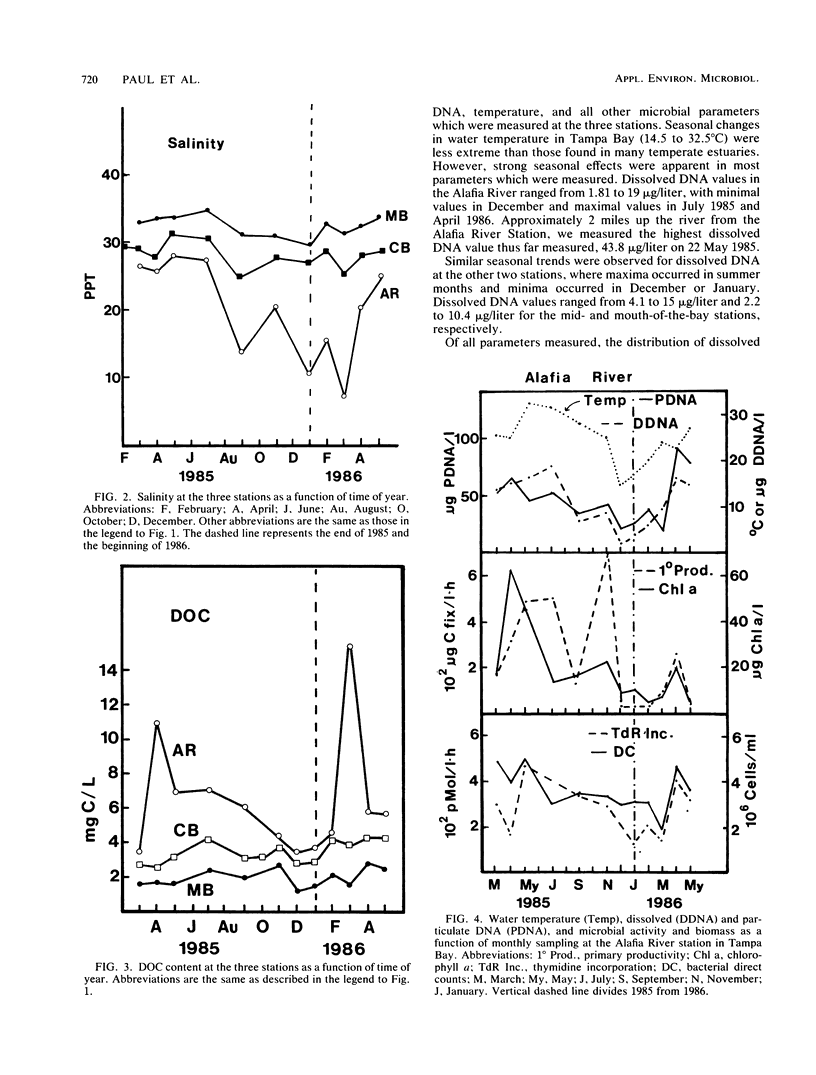
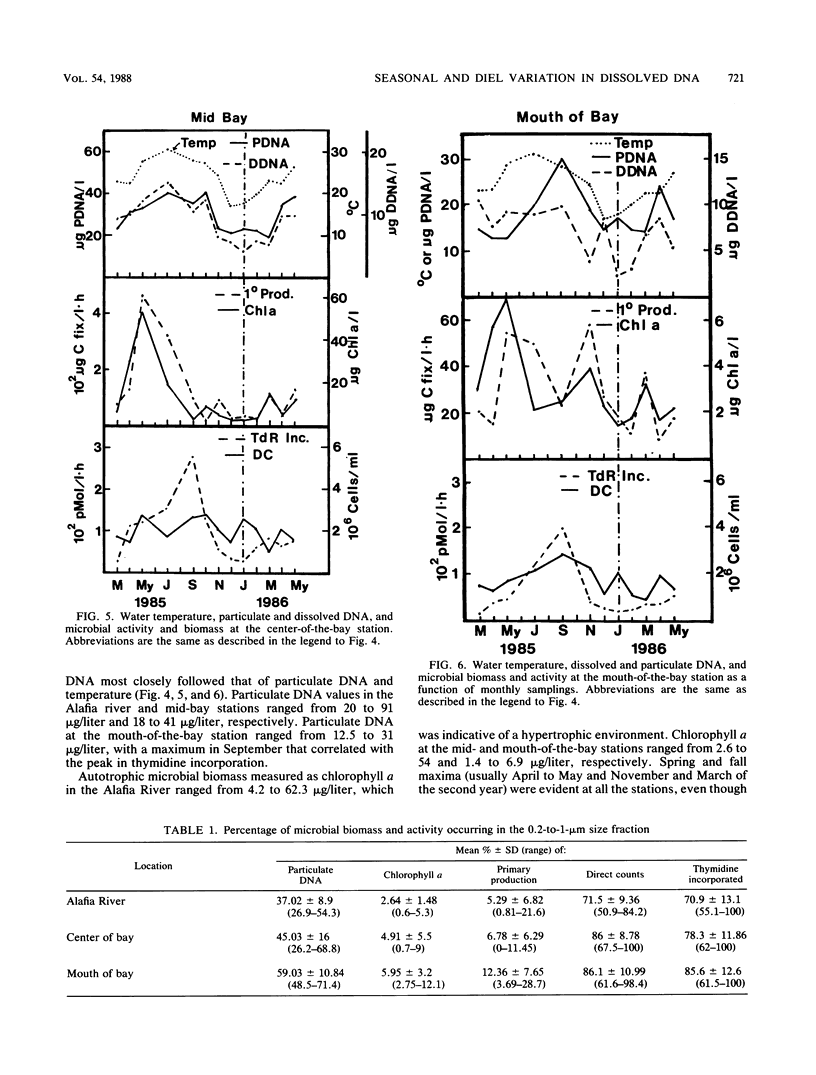
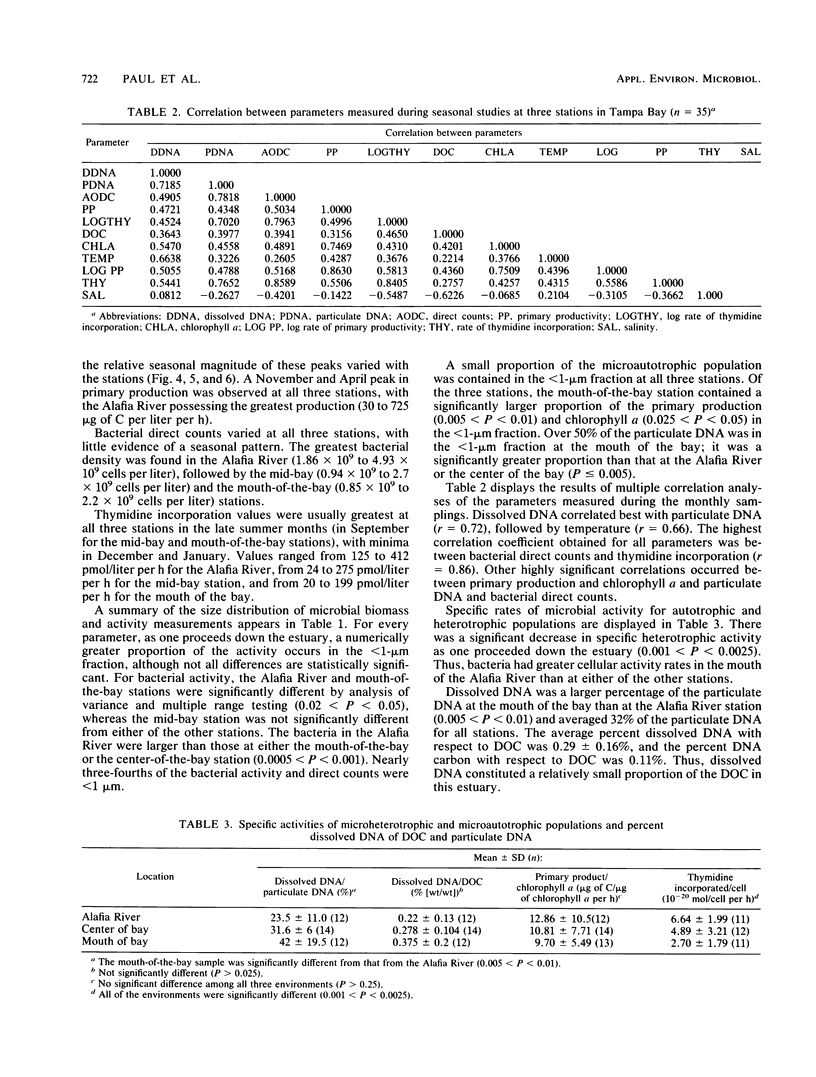
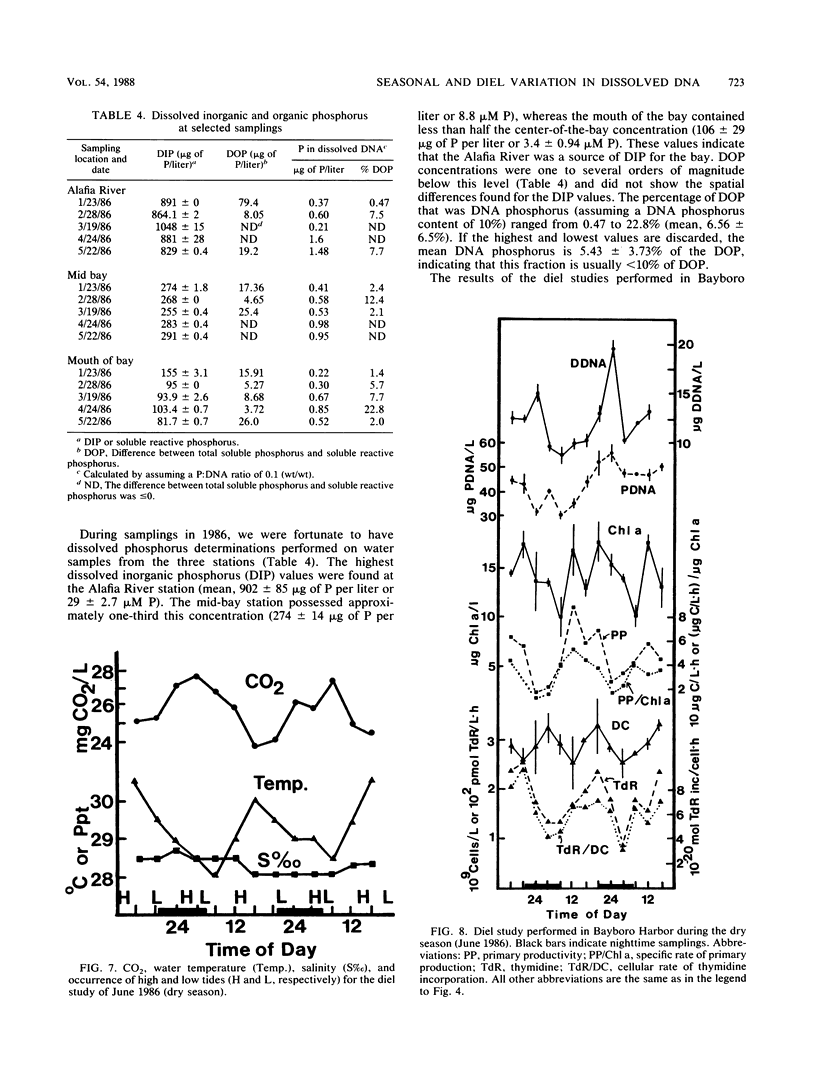
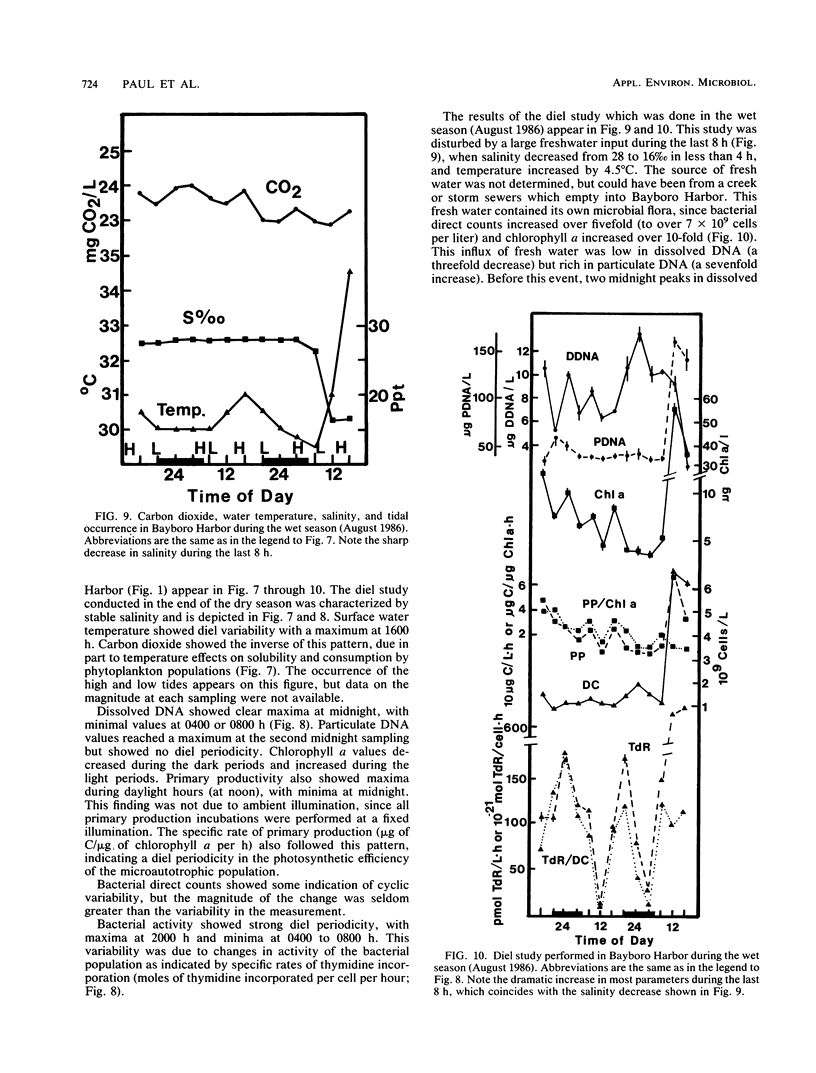
Abstract
Dissolved DNA and microbial biomass and activity parameters were measured over a 15-month period at three stations along a salinity gradient in Tampa Bay, Fla. Dissolved DNA showed seasonal variation, with minimal values in December and January and maximal values in summer months (July and August). This pattern of seasonal variation followed that of particulate DNA and water temperature and did not correlate with bacterioplankton (direct counts and [3H]thymidine incorporation) or phytoplankton (chlorophyll a and 14CO2 fixation) biomass and activity. Microautotrophic populations showed maxima in the spring and fall, whereas microheterotrophic activity was greatest in late summer (September). Both autotrophic and heterotrophic microbial activity was greatest at the high estuarine (low salinity) station and lowest at the mouth of the bay (high salinity station), irrespective of season. Dissolved DNA carbon and phosphorus constituted 0.11 ± 0.05% of the dissolved organic carbon and 6.6 ± 6.5% of the dissolved organic phosphorus, respectively. Strong diel periodicity was noted in dissolved DNA and in microbial activity in Bayboro Harbor during the dry season. A noon maximum in primary productivity was followed by an 8 p.m. maximum in heterotrophic activity and a midnight maximum in dissolved DNA. This diel periodicity was less pronounced in the wet season, when microbial parameters were strongly influenced by episodic inputs of freshwater. These results suggest that seasonal and diel production of dissolved DNA is driven by primary production, either through direct DNA release by phytoplankton, or more likely, through growth of bacterioplankton on phytoplankton exudates, followed by excretion and lysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deflaun M. F., Paul J. H., Davis D. Simplified method for dissolved DNA determination in aquatic environments. Appl Environ Microbiol. 1986 Oct;52(4):654–659. doi: 10.1128/aem.52.4.654-659.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. K., Rao D. V., Harrison W. G., Smith J. C., Cullen J. J., Irwin B., Platt T. Autotrophic picoplankton in the tropical ocean. Science. 1983 Jan 21;219(4582):292–295. doi: 10.1126/science.219.4582.292. [DOI] [PubMed] [Google Scholar]
- Meyer-Reil L. A. Seasonal and spatial distribution of extracellular enzymatic activities and microbial incorporation of dissolved organic substrates in marine sediments. Appl Environ Microbiol. 1987 Aug;53(8):1748–1755. doi: 10.1128/aem.53.8.1748-1755.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jeffrey W. H., DeFlaun M. F. Dynamics of extracellular DNA in the marine environment. Appl Environ Microbiol. 1987 Jan;53(1):170–179. doi: 10.1128/aem.53.1.170-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of hoechst 33258. Appl Environ Microbiol. 1982 Jun;43(6):1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B., Søndergaard M. Measurements of diel rates of bacterial secondary production in aquatic environments. Appl Environ Microbiol. 1984 Apr;47(4):632–638. doi: 10.1128/aem.47.4.632-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


