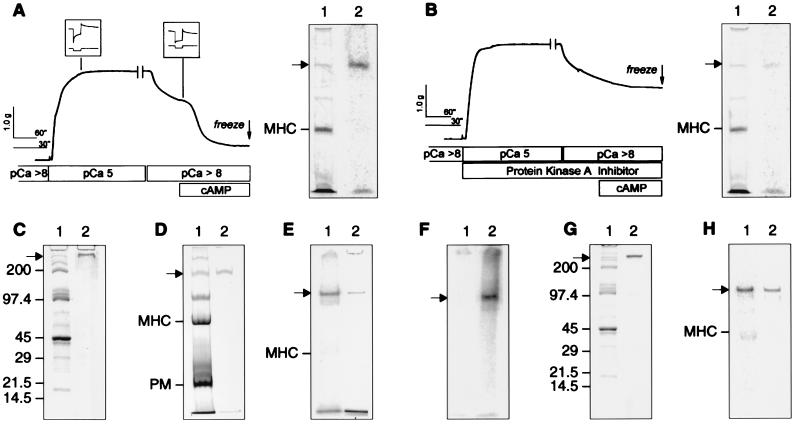
Figure 1.
Mechanical responses of the permeabilized ABRM during catch and the control of the release of catch by cAMP-dependent phosphorylation of a high molecular weight protein. (A) ABRM was activated in pCa 5 for 5 min, then washed in pCa >8 containing 20 mM EGTA. (Inset) Force responses (upper trace) to quick release in length of 5% Lo (lower trace), recorded for 10 sec after release. Addition of 100 μM cAMP causes rapid relaxation. γ32P-ATP was included for the last 3.5 min of pCa 5 onward. Muscle was frozen 1 min after addition of cAMP. The break in the force trace indicates expansion of subsequent time scale. Also shown is Coomassie blue-stained 4% acrylamide gel (lane 1) containing total muscle protein, and its PhosphorImage (lane 2). (B) ABRM was subjected to same protocol as in A, except that peptide inhibitor of protein kinase A (10 μg/ml) was included. (C–E) 4–20%, 6%, and 4% acrylamide gels, respectively, containing the total protein from the ABRM (lane 1) and the protein purified as described in the text (lane 2). All are Coomassie blue-stained, except E (lane 1), which shows a PhosphorImage of an ABRM strip treated with cAMP and γ32P-ATP. (F) A PhosphorImage of a 4% acrylamide gel containing the isolated protein treated with the catalytic subunit of protein kinase A and γ32P-ATP in the presence (lane 1) and absence (lane 2) of a peptide inhibitor of the kinase. (G) A 4–20% acrylamide gradient gel of the total protein extract from the ABRM either stained with Coomassie blue (lane 1) or subjected to Western blotting with the antipeptide antibody (lane 2). (H) A PhosphorImage (lane 1) and a Western blot using the anti-peptide antibody (lane 2) from a 5% acrylamide gel containing protein from a permeabilized muscle, treated as described in A. MHC, myosin heavy chain; PM, paramyosin. → shows the position of the CR protein.