Abstract
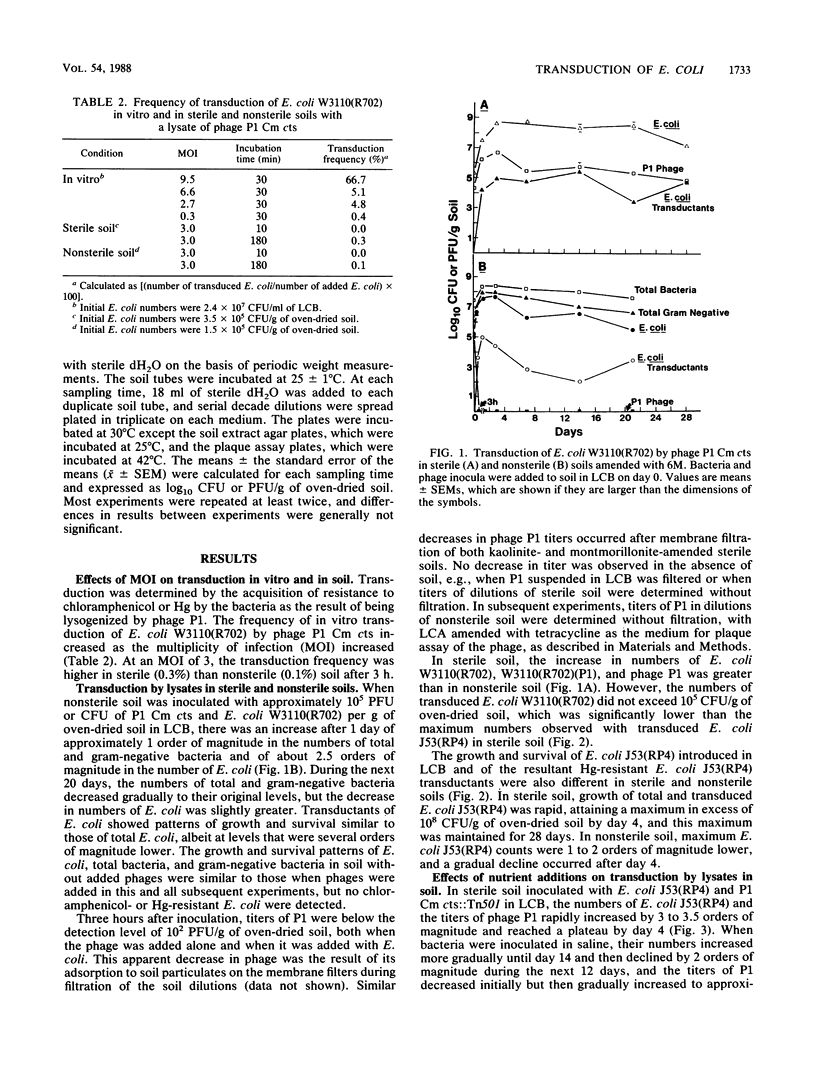
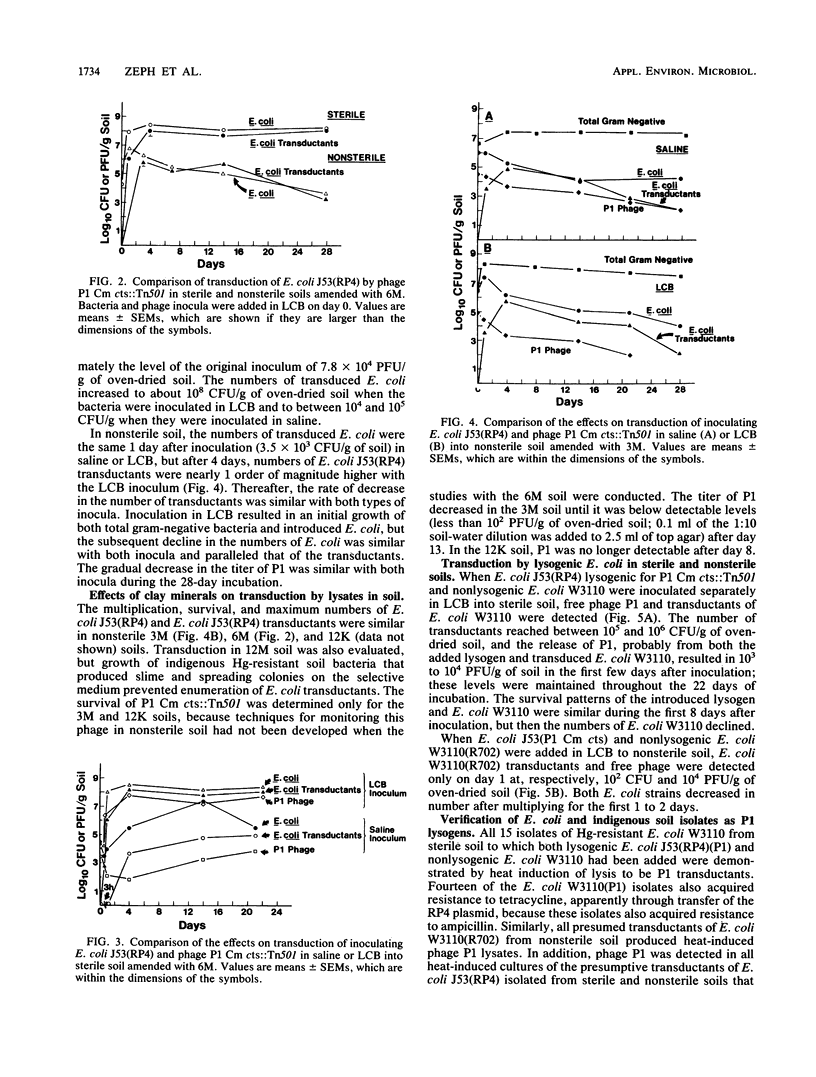
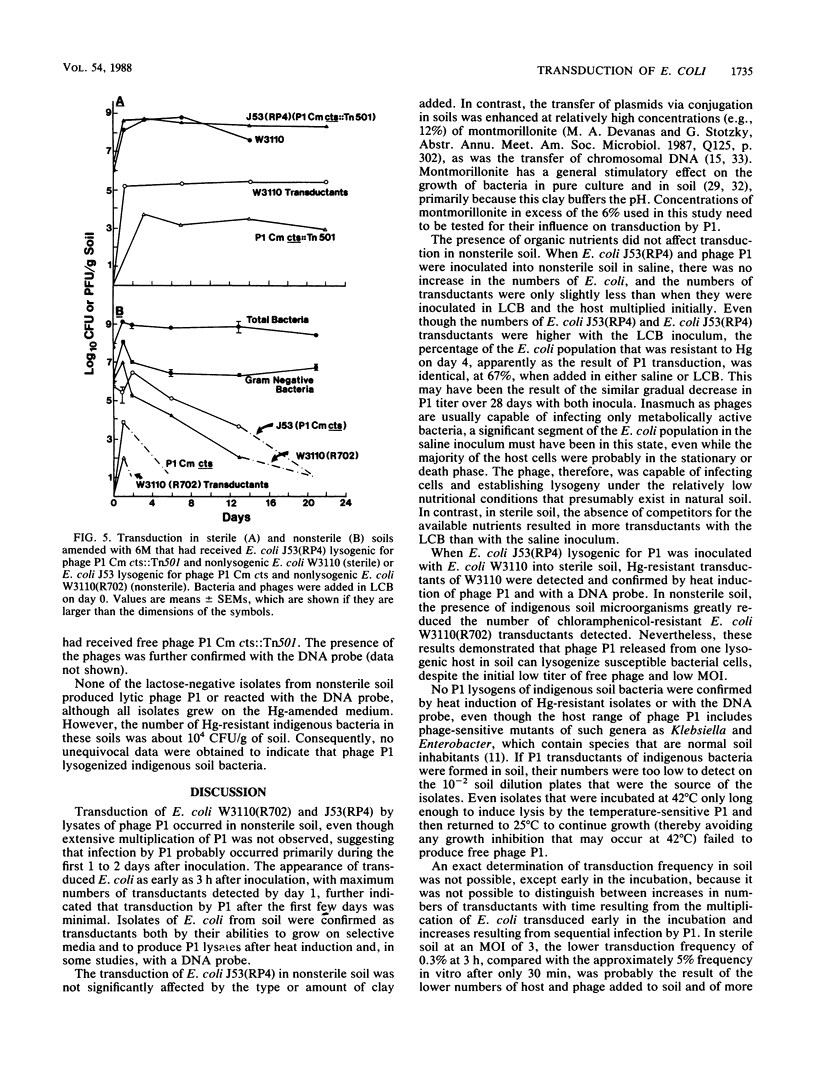
Transduction of Escherichia coli W3110(R702) and J53(RP4) (10(4) to 10(5) CFU/g of soil) by lysates of temperature-sensitive specialized transducing derivatives of bacteriophage P1 (10(4) to 10(5) PFU/g of soil) (P1 Cm cts, containing the resistance gene for chloramphenicol, or P1 Cm cts::Tn501, containing the resistance genes for chloramphenicol and mercury [Hg]) occurred in soil amended with montmorillonite or kaolinite and adjusted to a -33-kPa water tension. In nonsterile soil, survival of introduced E. coli and the numbers of E. coli transductants resistant to chloramphenicol or Hg were independent of the clay amendment. The numbers of added E. coli increased more when bacteria were added in Luria broth amended with Ca and Mg (LCB) than when they were added in saline, and E. coli transductants were approximately 1 order of magnitude higher in LCB; however, the same proportion of E. coli was transduced with both types of inoculum. In sterile soil, total and transduced E. coli and P1 increased by 3 to 4 logs, which was followed by a plateau when they were inoculated in LCB and a gradual decrease when they were inoculated in saline. Transduction appeared to occur primarily in the first few days after addition of P1 to soil. The transfer of Hg or chloramphenicol resistance from lysogenic to nonlysogenic E. coli by phage P1 occurred in both sterile and nonsterile soils. On the basis of heat-induced lysis and phenotype, as well as hybridization with a DNA probe in some studies, the transductants appeared to be the E. coli that was added. Transduction of indigenous soil bacteria was not unequivocally demonstrated.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich H., Stotzky G. Effect of cadmium on fungi and on interactions between fungi and bacteria in soil: influence of clay minerals and pH. Appl Environ Microbiol. 1977 May;33(5):1059–1066. doi: 10.1128/aem.33.5.1059-1066.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystricky V., Stotzky G., Schiffenbauer M. Electron microscopy of T1-bacteriophage adsorbed to clay minerals: application of the critical point drying method. Can J Microbiol. 1975 Aug;21(8):1278–1282. doi: 10.1139/m75-192. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic manipulation of microorganisms: potential benefits and biohazards. Annu Rev Microbiol. 1976;30:507–533. doi: 10.1146/annurev.mi.30.100176.002451. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Bender R. A., Streicher S. L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974 Jun;118(3):810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W. R factors from Proteus mirabilis and P. vulgaris. J Gen Microbiol. 1975 Apr;87(2):301–311. doi: 10.1099/00221287-87-2-301. [DOI] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Transfer of erythromycin resistance from clinically isolated lysogenic strains of Streptococcus pyogenes via their endogenous phage. J Infect Dis. 1978 Sep;138(3):281–286. doi: 10.1093/infdis/138.3.281. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance plasmids of Staphylococcus aureus and their clinical importance. Bacteriol Rev. 1975 Mar;39(1):1–32. doi: 10.1128/br.39.1.1-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W. D., Miller R. V., Sayler G. S. Frequency of F116-mediated transduction of Pseudomonas aeruginosa in a freshwater environment. Appl Environ Microbiol. 1978 Nov;36(5):724–730. doi: 10.1128/aem.36.5.724-730.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Morse S. I. In vivo transmission of drug resistance factors between strains of Staphylococcus aureus. J Exp Med. 1967 Jan 1;125(1):45–59. doi: 10.1084/jem.125.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Construction of haloaromatics utilising bacteria. Nature. 1979 Feb 1;277(5695):385–386. doi: 10.1038/277385a0. [DOI] [PubMed] [Google Scholar]
- Rissler J. F. Research needs for biotic environmental effects of genetically-engineered microorganisms. Recomb DNA Tech Bull. 1984 Mar;7(1):20–30. [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Saye D. J., Ogunseitan O., Sayler G. S., Miller R. V. Potential for transduction of plasmids in a natural freshwater environment: effect of plasmid donor concentration and a natural microbial community on transduction in Pseudomonas aeruginosa. Appl Environ Microbiol. 1987 May;53(5):987–995. doi: 10.1128/aem.53.5.987-995.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffenbauer M., Stotzky G. Adsorption of coliphages T1 and T7 to clay minerals. Appl Environ Microbiol. 1982 Mar;43(3):590–596. doi: 10.1128/aem.43.3.590-596.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples F. E. Spread of organisms with novel genotypes: thoughts from an ecological perspective. Recomb DNA Tech Bull. 1983 Jun;6(2):43–56. [PubMed] [Google Scholar]
- Stotzky G., Babich H. Survival of, and genetic transfer by, genetically engineered bacteria in natural environments. Adv Appl Microbiol. 1986;31:93–138. doi: 10.1016/s0065-2164(08)70440-4. [DOI] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985 Jan;49(1):19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


