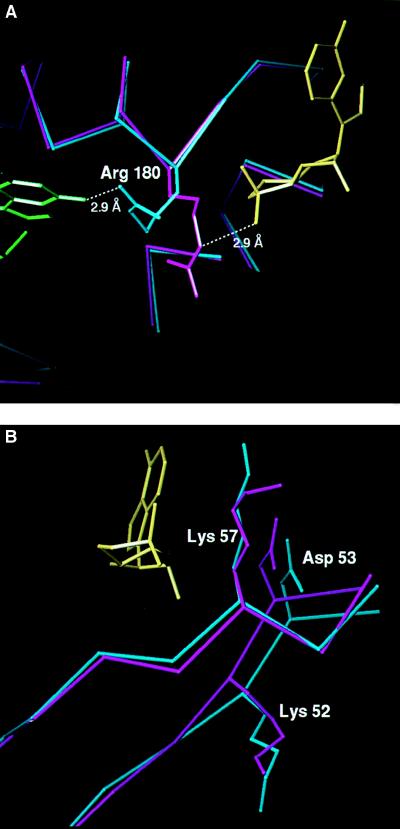
Figure 4.
Comparison of part of the DNA binding domain (A) and the β-hairpin loop (B) structures of CAP complexed with only one cAMP per subunit (magenta) with that of CAP complexed with DNA and two cAMP molecules per subunit (cyan). (A) α-Carbon backbones of the small domains were superimposed. In the structure of CAP complexed with only one cAMP per subunit and without DNA, Arg-180 could hydrogen-bond with the phosphate of the syn-cAMP (yellow); whereas in the structure of CAP complexed with DNA and two cAMP molecules per subunit, Arg-180 hydrogen bonds to guanosine-7. (B) α-Carbon backbones of the large domains, excluding the β-hairpin loop, were superimposed, and the side chains for Lys-52, Asp-53, and Lys-57 are shown.