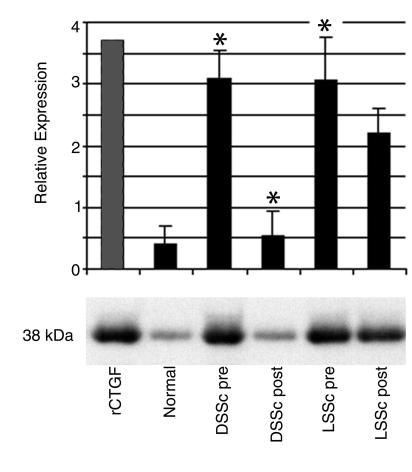
Figure 8.
Forearm skin blister fluid was obtained from six healthy control subjects, from the lesional skin of six diffuse scleroderma patients before and after 5 days of Iloprost therapy, and from the lesional skin of six limited scleroderma patients before and after 5 days of Iloprost therapy. Blister fluid CTGF was measured by Western blot analysis. rCTGF, recombinant CTGF (200 ng/ml); Normal, healthy controls; DSSc pre, diffuse scleroderma patients before Iloprost; DSSc post, diffuse scleroderma patients after Iloprost treatment; LSSc pre, limited scleroderma patients before Iloprost treatment; and LSSc post, limited scleroderma patients after Iloprost treatment. *P < 0.01.