Figure 2.
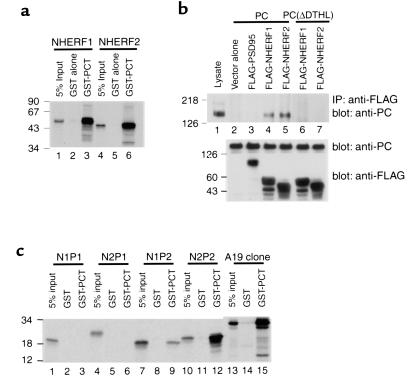
PC associates with the second PDZ domain of NHERF proteins through its C-terminal PDZ-binding motif. (a) Interaction of GST-PCT with NHERF proteins. NHERF2 bind specifically to GST-PCT but not to GST alone (lanes 2 and 5), as well as NHERF1 (lane 3). Equivalent amounts of GST-PCT (7 μg) or GST alone (∼5 μg) bound to glutathione-agarose beads were incubated with in vitro–translated, radiolabeled NHERF1 or NHERF2. The precipitates were separated by 15% SDS-PAGE and detected by autoradiography. 5% input, 5% of the in vitro–translated products. (b) PC binds NHERF proteins, and the C-terminus of PC is essential for the interaction. PC coimmunoprecipitates with both NHERF proteins (lanes 4 and 5), but not with vector alone (lane 2) or with PSD95 (lane 3). PC, lacking the last four C-terminal residues [PC(ΔDTHL)], does not associate with either NHERF protein (lanes 6 and 7). Lane 1: 10 μg cell lysate. PC or PC(ΔDTHL) were transiently coexpressed in Cos 7 cells with either NHERF1, NHERF2, or PSD95 (control) followed by immunoprecipitation with anti-FLAG (M2). The precipitated proteins were immunoblotted with anti-PC (0601). The middle and lower panels demonstrate that the transfected cells express PC, PC mutant, and NHERF at comparable levels. (c) PDZ2 of both NHERF proteins specifically associates with GST-PCT (lanes 9 and 12). The A19 clone (see Figure 1a) is used as a positive control. Neither PDZ1 domain (lanes 3 and 6) associates with GST-PCT. Experiment performed as in a. Size markers indicate molecular mass in kilodaltons.