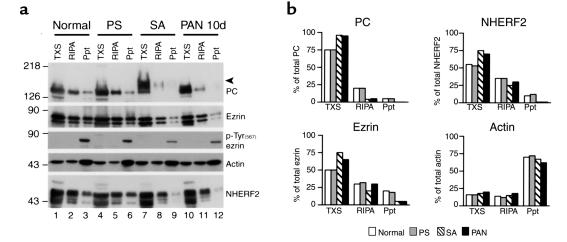
Figure 9.
Dissociation of PC/NHERF2/ezrin complexes from the cytoskeleton in glomeruli from SA- and PAN-treated rats. (a) Immunoblot of normal, PS-, SA-, or PAN-treated (10d) rats. In normal glomeruli, PC, NHERF2, and ezrin distribute in all three fractions — that is, Triton X-100 soluble (TXS), RIPA soluble (RIPA), and RIPA insoluble (Ppt). In SA- and PAN-, but not PS-treated glomeruli, there is a significant increase in the amount of PC, NHERF2, and ezrin found in the Triton X-100 soluble (TXS) fraction. Actin is also distributed in all three fractions, but its pattern of distribution is unchanged among these experimental groups. C-terminal threonine phosphorylated ezrin [ezrin, pTyr(567)] is present only in the insoluble precipitate (Ppt) (lanes 3, 6, 9, and 12) under all conditions. The level of phosphorylated ezrin is significantly reduced in SA- and PAN-treated glomeruli. Isolated glomeruli from normal, PS-, SA-, and PAN-treated (10d) rats were solubilized sequentially with 0.5% Triton X-100 and RIPA lysis buffer and separated into Triton X-100–soluble (TXS), RIPA-soluble (RIPA), and RIPA-insoluble fractions (Ppt). Equal volumes of these fractions were immunoblotted with appropriate Ab’s. The arrowhead indicates the position of desialylated PC (lane 7). The experiment was performed twice with comparable results. (b) Quantification of protein bands shown in a was done by densitometry as described in Methods.