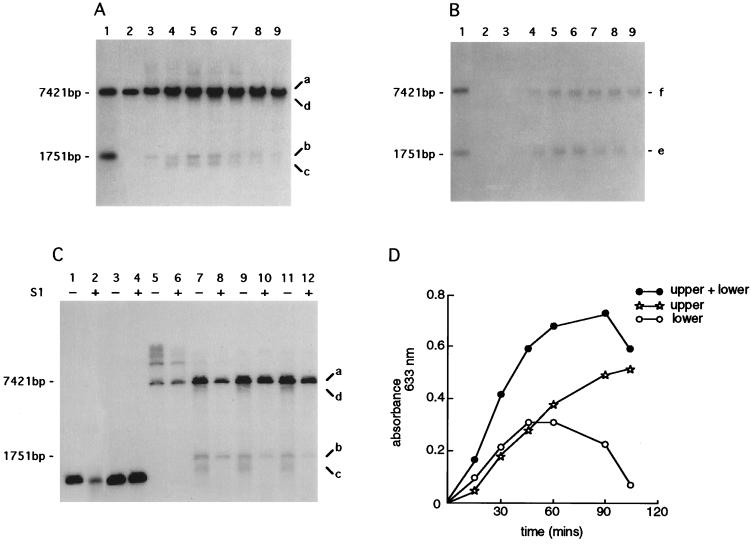
Figure 3.
Time course of the formation of ssDNA intermediates. An infection was established by wild-type λ (STU4) in strain FA77 (rec+) carrying pPAORM3.8. Cells were harvested at 15-min intervals; total DNA extracts were prepared and digested in vitro with EcoRI. Gels were blotted onto nitrocellulose membranes under either Southern conditions (i.e., with denaturation) or native conditions (i.e., without denaturation). (A) Southern blot analysis of total DNA extracts digested in vitro. (B) Native blot of the same DNA samples as used in A. Lane 1, size standards for EcoRI digestion (7421 bp) and EcoRI/XhoI double digestion (1751 bp) of total λ DNA (see restriction map, Fig. 2); lanes 2–9, samples harvested at 15-min intervals; lane 2, time 0; lane 3, 15 min; lane 4, 30 min; lane 5, 45 min; lane 6, 60 min; lane 7, 75 min; lane 8, 90 min; and lane 9, 105 min. Each blot was probed with oligonucleotide 54 (position indicated in Fig. 2) designed to hybridize with single-strand overhangs of 3′ polarity. Signals designated a, b, c, d, e, and f indicate DNA intermediates referred to in the text. (C) Southern analysis of DNAs treated with S1 nuclease. DNA samples were either untreated (−) or treated (+) with 1 unit of S1 nuclease, except for lane 2, which was treated with 10 units. Lanes 1, 2, 3, and 4, represent a gel-purified XhoI/PstI λ fragment and serve as the control for nuclease digestion; lanes 5–12, samples harvested at 15-min intervals and digested in vitro with EcoRI; lanes 5 and 6, time 0; lanes 7 and 8, 15 min; lanes 9 and 10, 30 min; and lanes 11 and 12, 60 min. The blot was probed with oligonucleotide 54. Signals designated a, b, c, and d correspond to those in A. (D) Densitometric scanning of the native blot presented in B. “Lower” refers to the signal at 1751 bp; “upper” refers to the signal at 7421 bp; “upper + lower” indicates the sum of the two signals.