Abstract
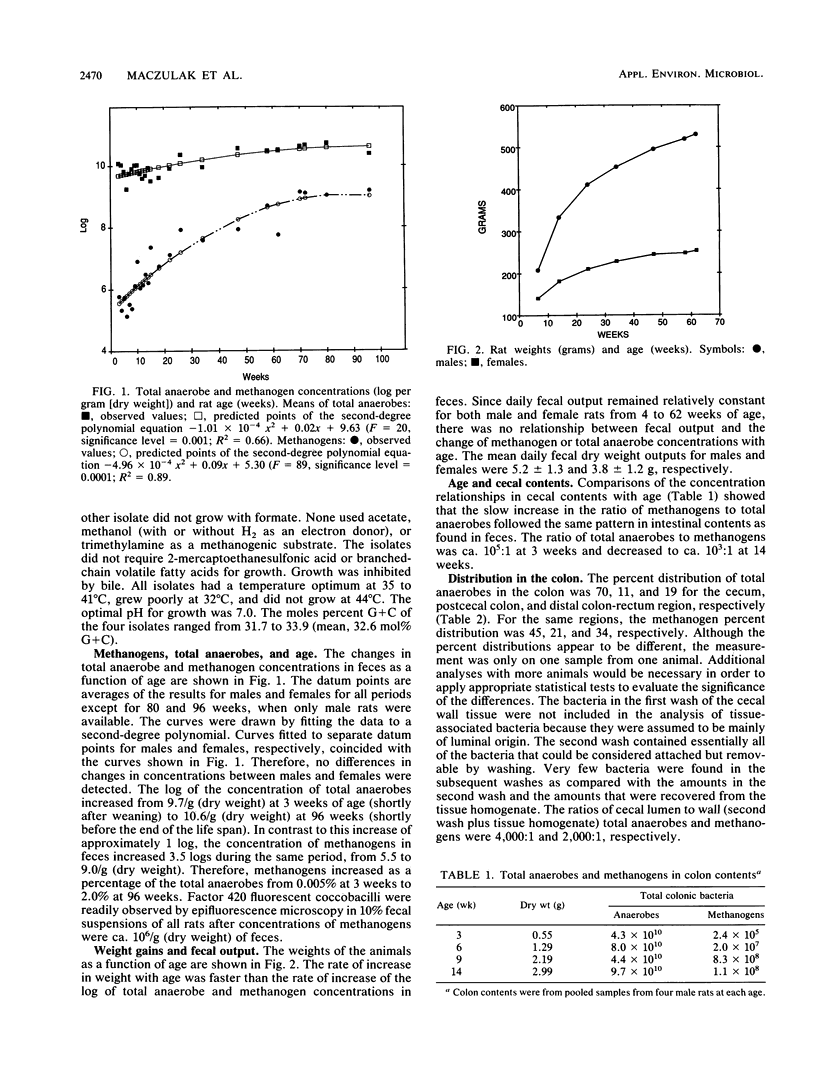
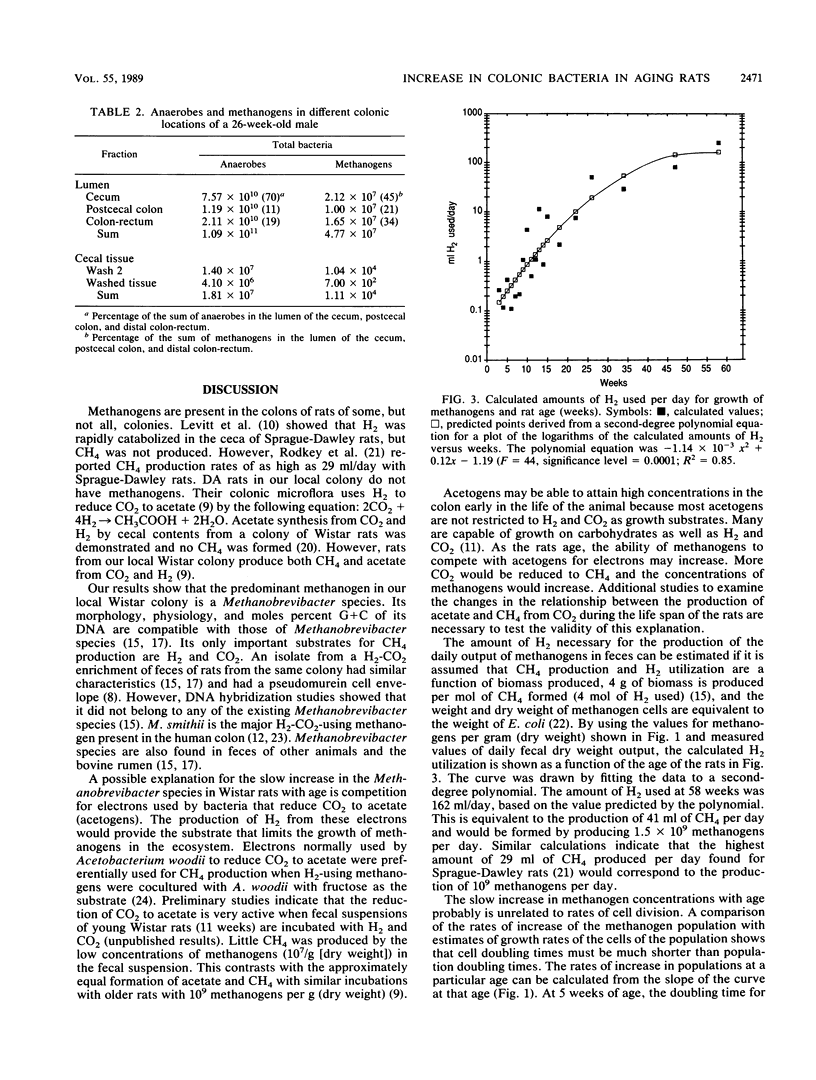
Methanogens are present in the colons of our local Wistar rat colony. We studied the changes in concentrations of their fecal methanogenic and nonmethanogenic bacteria with age as a model of the development of these communities in humans. We found that the predominant methanogen in the rats is a Methanobrevibacter species. The log of the concentration of total anaerobes increased from 9.8/g (dry weight) at 3.0 weeks of age (shortly after weaning) to 10.7/g (dry weight) at 96 weeks (shortly before the end of the life span). In contrast, the log concentration of methanogens increased from 5.5 to 9/g (dry weight) during the same time period. Therefore, methanogens increased as a percentage of the total anaerobes from 0.005% at 3.0 weeks to 2.0% at 96 weeks. About 12 doublings of the methanogenic population and 3.3 doublings of the nonmethanogenic population took place from weaning until death. The slow increase in the ratio of methanogens to total anaerobes with age followed the same pattern in cecal contents as found in feces. There were no relationships between animal weights or fecal outputs and the increase in total anaerobe and methanogen concentrations in feces. A possible explanation for the slow increase in the Methanobrevibacter species in Wistar rats with age is a gradual shifting of the use of electrons from the reduction of CO2 to acetate by acetogens to the reduction of CO2 to CH4. The results provide the first evidence for an age-related change in the nonmethanogenic bacteria of the colon and supporting microbiological evidence for physiological studies that have shown age-related increases in colonic methane production in humans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. H., Jr, Engel R. R., Levitt M. D. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971 Mar 1;133(3):572–588. doi: 10.1084/jem.133.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Influence of CH4 production by Methanobacterium ruminantium on the fermentation of glucose and lactate by Selenomonas ruminantium. Appl Environ Microbiol. 1977 Dec;34(6):756–759. doi: 10.1128/aem.34.6.756-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel J. F., Flourie B., Neut C., Florent C., Leblond A., Rambaud J. C. La méthanogenèse chez l'homme. Gastroenterol Clin Biol. 1987 Oct;11(10):694–700. [PubMed] [Google Scholar]
- Demigné C., Rémésy C. Stimulation of absorption of volatile fatty acids and minerals in the cecum of rats adapted to a very high fiber diet. J Nutr. 1985 Jan;115(1):53–60. doi: 10.1093/jn/115.1.53. [DOI] [PubMed] [Google Scholar]
- Lajoie S. F., Bank S., Miller T. L., Wolin M. J. Acetate production from hydrogen and [13C]carbon dioxide by the microflora of human feces. Appl Environ Microbiol. 1988 Nov;54(11):2723–2727. doi: 10.1128/aem.54.11.2723-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. D., Berggren T., Hastings J., Bond J. H. Hydrogen (H2) catabolism in the colon of the rat. J Lab Clin Med. 1974 Aug;84(2):163–167. [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J., Conway de Macario E., Macario A. J. Isolation of Methanobrevibacter smithii from human feces. Appl Environ Microbiol. 1982 Jan;43(1):227–232. doi: 10.1128/aem.43.1.227-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol. 1982 Feb;131(1):14–18. doi: 10.1007/BF00451492. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Stability of Methanobrevibacter smithii populations in the microbial flora excreted from the human large bowel. Appl Environ Microbiol. 1983 Jan;45(1):317–318. doi: 10.1128/aem.45.1.317-318.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y., Miyaki K. Effects of age and starvation on the gastrointestinal microflora and the heat resistance of fecal bacteria in rats. Microbiol Immunol. 1979;23(6):455–470. doi: 10.1111/j.1348-0421.1979.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Peled Y., Gilat T., Liberman E., Bujanover Y. The development of methane production in childhood and adolescence. J Pediatr Gastroenterol Nutr. 1985 Aug;4(4):575–579. doi: 10.1097/00005176-198508000-00013. [DOI] [PubMed] [Google Scholar]
- Rodkey F. L., Collison H. A., O'Neal J. D. Carbon monoxide and methane production in rats, guinea pigs, and germ-free rats. J Appl Physiol. 1972 Aug;33(2):256–260. doi: 10.1152/jappl.1972.33.2.256. [DOI] [PubMed] [Google Scholar]
- Weaver G. A., Krause J. A., Miller T. L., Wolin M. J. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986 Jun;27(6):698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw A., Sleath K. Myth of the marsupial mother: home care of very low birth weight babies in Bogota, Colombia. Lancet. 1985 May 25;1(8439):1206–1208. doi: 10.1016/s0140-6736(85)92877-6. [DOI] [PubMed] [Google Scholar]
- Winter J. U., Wolfe R. S. Methane formation from fructose by syntrophic associations of Acetobacterium woodii and different strains of methanogens. Arch Microbiol. 1980 Jan;124(1):73–79. doi: 10.1007/BF00407031. [DOI] [PubMed] [Google Scholar]


