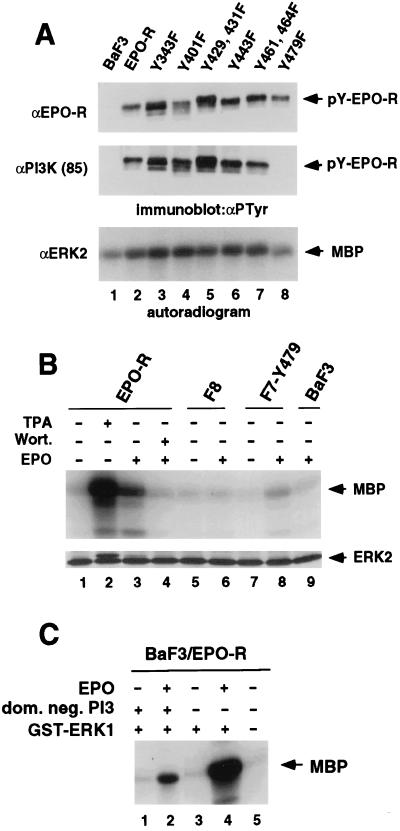
Figure 2.
Tyr-479 is essential for association of the EPO-R with the p85 subunit of PI3 kinase and is important for MAPK activation. (A) Parental BaF3 cells and BaF3 cells expressing the wild-type EPO-R or the various mutant EPO-Rs were stimulated with EPO, lysed, and used for immunoprecipitation with antisera directed against the EPO-R, the regulatory subunit of PI3 kinase (p85), or ERK2 as indicated. The tyrosine phosphorylated form of the immunoprecipitated receptor (pY-EPO-R) was detected by immunoblotting with the monoclonal anti-PTyr antibody 4G10 (αPTyr) and is indicated by arrows (Top and Middle). MAPK immunoprecipitated was subjected to an in vitro kinase assay using [γ-32P]ATP. Phosphorylation of the exogenous substrate MBP was detected by autoradiography and is marked by an arrow (Bottom). (B) Tyr-479 is sufficient for activation of MAPK. Prior to the MAPK assays, parental BaF3 cells and BaF3 cells expressing the wild-type EPO-R or mutant EPO-Rs F8 or F7Y479 were starved as described in Materials and Methods and either left unstimulated (−) or treated for 5 min with 100 units EPO per ml (+) prior to lysis. Stimulation for 15 min with 200 nM TPA in the absence of EPO and pre treatment of cells with 100 nM wortmannin in the presence of EPO are indicated (+). MAPK was immunoprecipitated by an antiserum directed against ERK2 and subjected to an in vitro kinase assay using [γ-32P]ATP. Phosphorylation of the exogenous substrate MBP was detected by autoradiography and equal immunoprecipitation of the ERK2 MAPK was confirmed by immunoblotting with anti-ERK2. The electrophoretic mobility shift of ERK2 detected in lane 2 and faintly in lanes 3 and 8 indicates the activation of MAPK due to phosphorylation. Similar results (not shown) were obtained when an antibody directed against ERK1 was used for immunoprecipitation. (C) An expression vector encoding a GST-ERK1 fusion protein (+) was transiently cotransfected either with an expression vector encoding a dominant negative form of PI3 kinase (dom. neg. PI3) (+) or a control vector into BaF3 cells expressing the EPO-R (BaF3/EPO-R). Twenty-four hours after transfection cells were starved for 4 hr as described and either left unstimulated (−) or treated for 5 min with 100 units EPO per ml (+). The GST-ERK1 fusion protein was precipitated from the lysates by absorption to glutathione agarose beads (Sigma) and subjected to an in vitro MAPK assay as described in Materials and Methods. Each transfection was done in triplicate and the autoradiogram of one representative experiment is shown.