Abstract
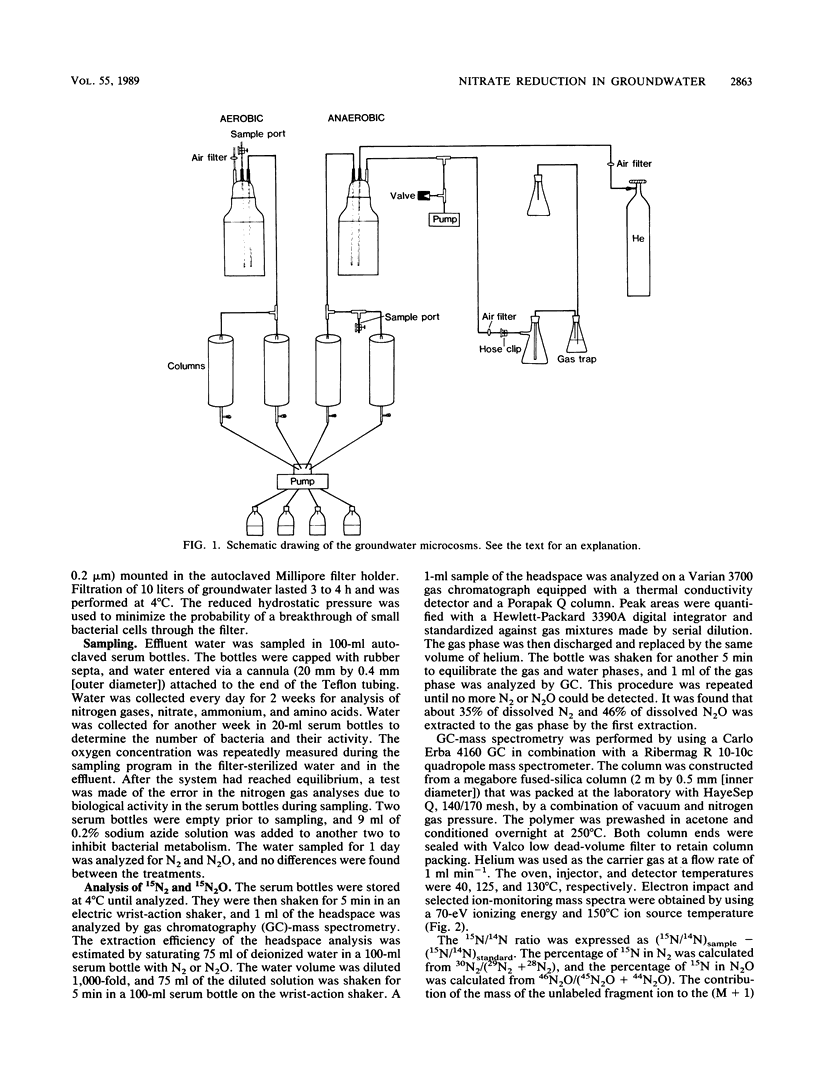
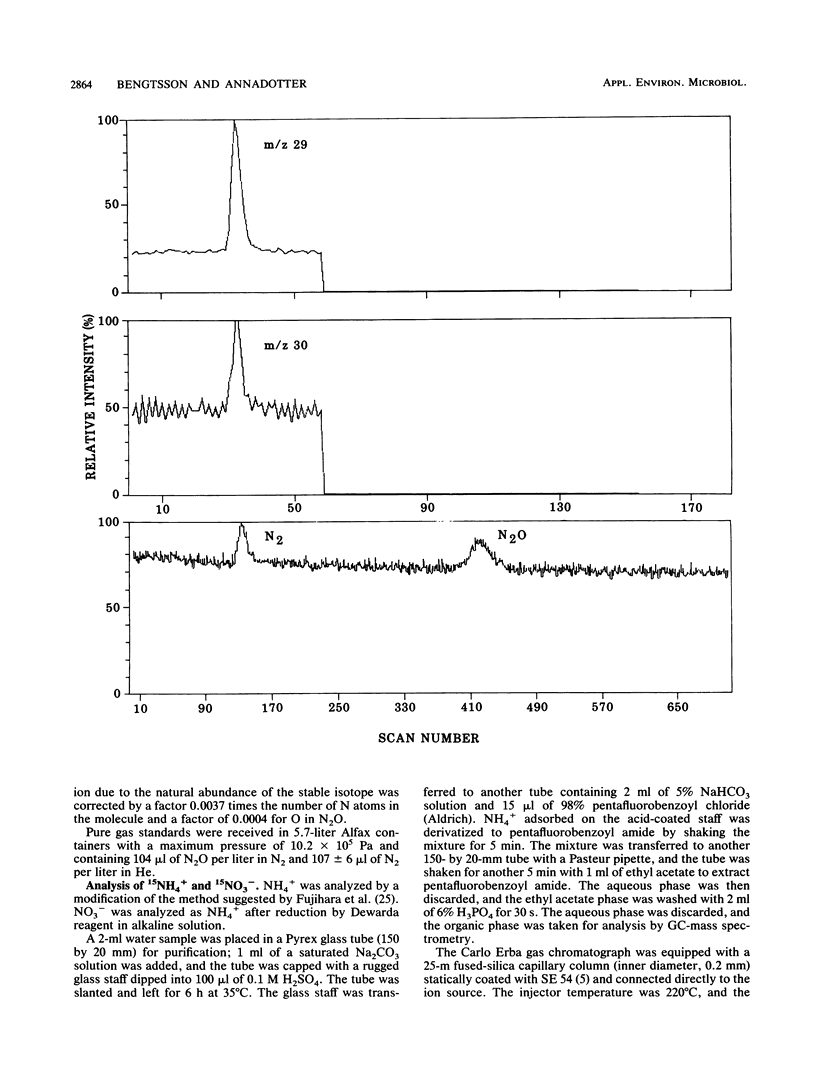
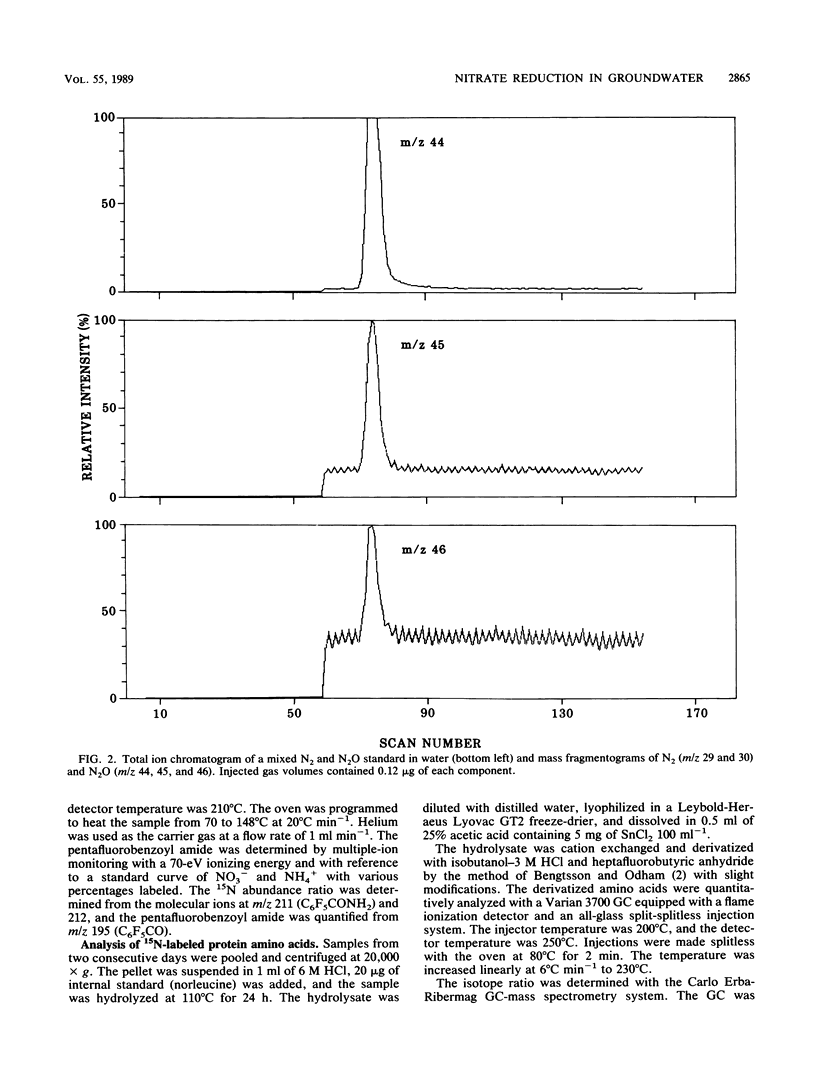
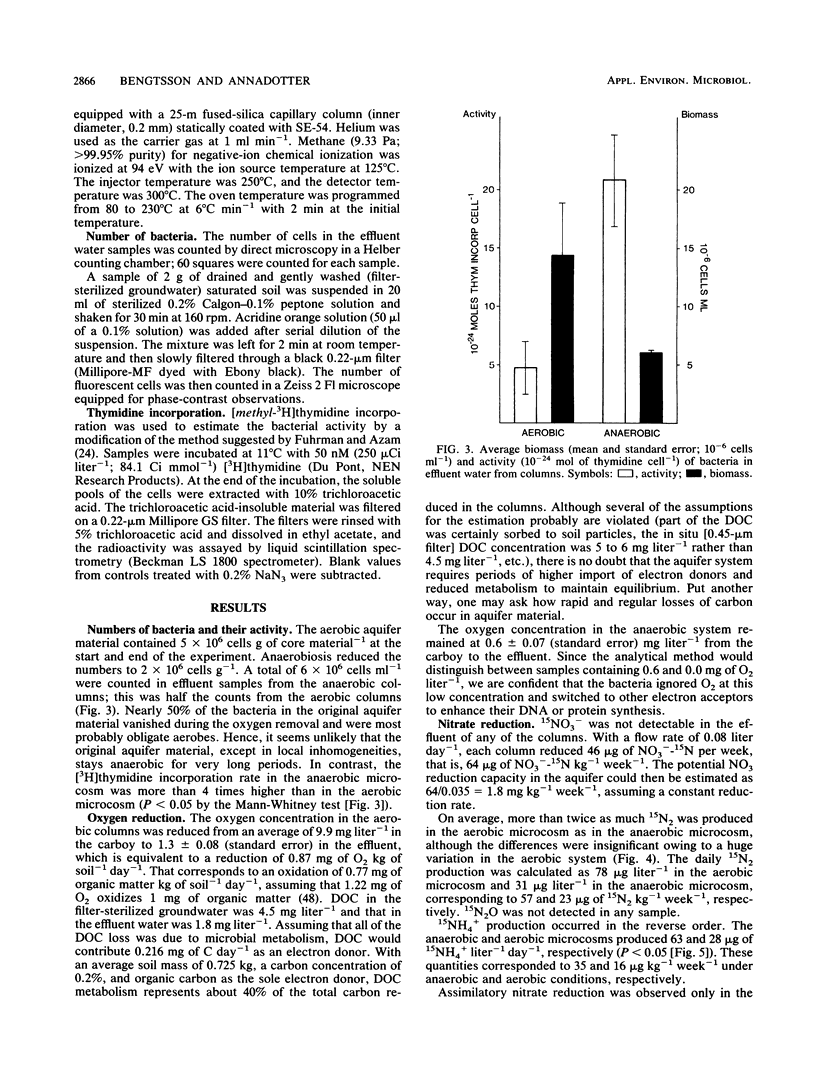
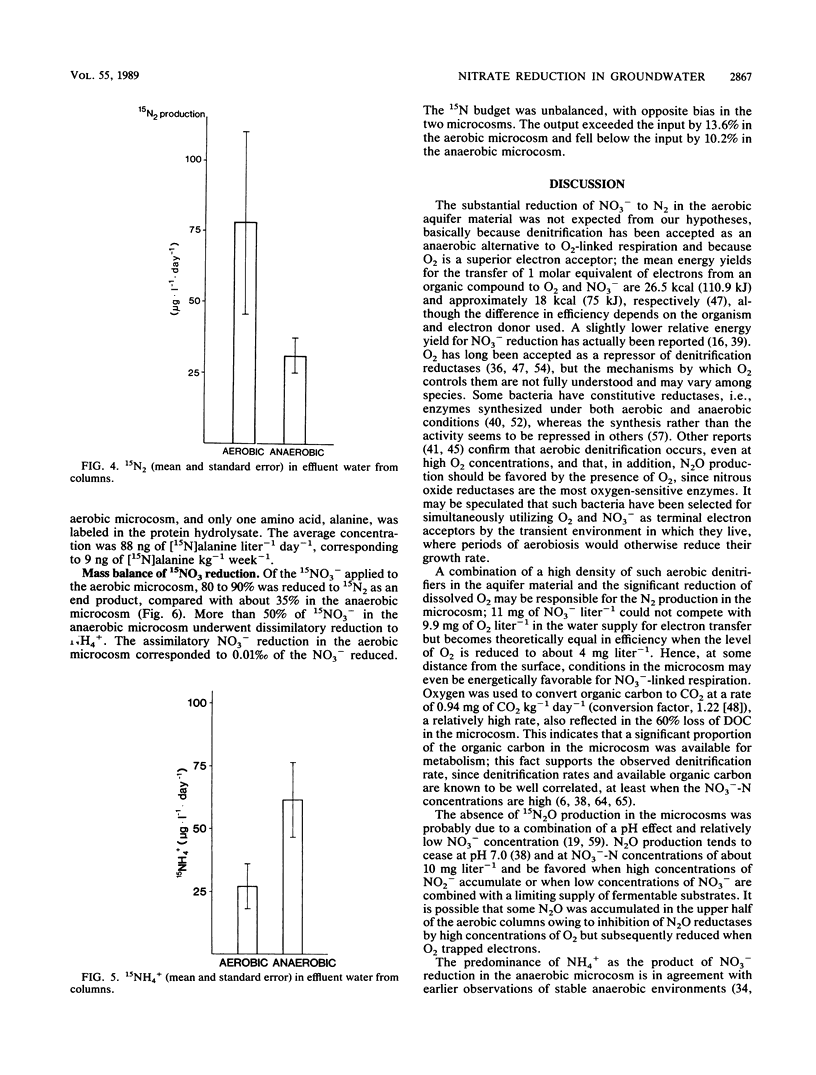
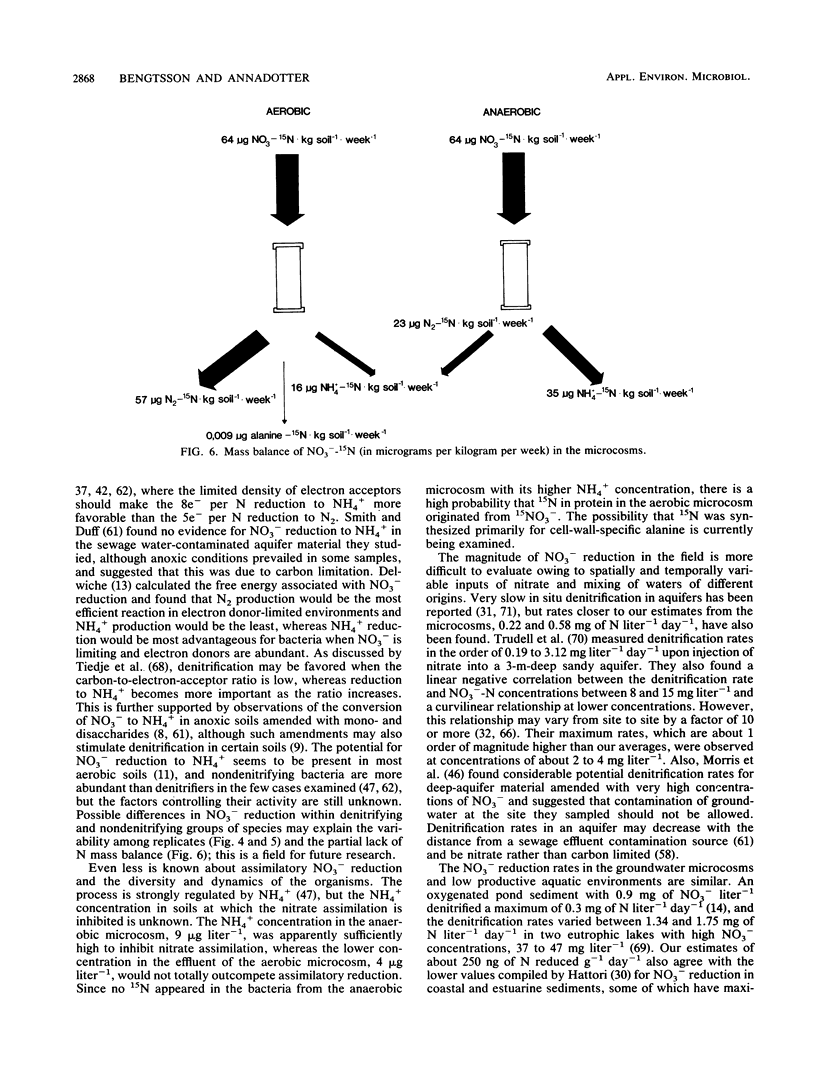
Aerobic and anaerobic groundwater continuous-flow microcosms were designed to study nitrate reduction by the indigenous bacteria in intact saturated soil cores from a sandy aquifer with a concentration of 3.8 mg of NO3−-N liter−1. Traces of 15NO3− were added to filter-sterilized groundwater by using a Darcy flux of 4 cm day−1. Both assimilatory and dissimilatory reduction rates were estimated from analyses of 15N2, 15N2O, 15NH4+, and 15N-labeled protein amino acids by capillary gas chromatography-mass spectrometry. N2 and N2O were separated on a megabore fused-silica column and quantified by electron impact-selected ion monitoring. NO3− and NH4+ were analyzed as pentafluorobenzoyl amides by multiple-ion monitoring and protein amino acids as their N-heptafluorobutyryl isobutyl ester derivatives by negative ion-chemical ionization. The numbers of bacteria and their [methyl-3H]thymidine incorporation rates were simultaneously measured. Nitrate was completely reduced in the microcosms at a rate of about 250 ng g−1 day−1. Of this nitrate, 80 to 90% was converted by aerobic denitrification to N2, whereas only 35% was denitrified in the anaerobic microcosm, where more than 50% of NO3− was reduced to NH4+. Assimilatory reduction was recorded only in the aerobic microcosm, where N appeared in alanine in the cells. The nitrate reduction rates estimated for the aquifer material were low in comparison with rates in eutrophic lakes and coastal sediments but sufficiently high to remove nitrate from an uncontaminated aquifer of the kind examined in less than 1 month.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson G., Odham G. A micromethod for the analysis of free amino acids by gas chromatography and its application to biological systems. Anal Biochem. 1979 Jan 15;92(2):426–443. doi: 10.1016/0003-2697(79)90681-x. [DOI] [PubMed] [Google Scholar]
- Bleakley B. H., Tiedje J. M. Nitrous oxide production by organisms other than nitrifiers or denitrifiers. Appl Environ Microbiol. 1982 Dec;44(6):1342–1348. doi: 10.1128/aem.44.6.1342-1348.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitrous oxide from soil denitrification: factors controlling its biological production. Science. 1980 May 16;208(4445):749–751. doi: 10.1126/science.208.4445.749. [DOI] [PubMed] [Google Scholar]
- Fong K. L., Crysler C. S., Mico B. A., Boyle K. E., Kopia G. A., Kopaciewicz L., Lynn R. K. Dose-dependent pharmacokinetics of recombinant tissue-type plasminogen activator in anesthetized dogs following intravenous infusion. Drug Metab Dispos. 1988 Mar-Apr;16(2):201–206. [PubMed] [Google Scholar]
- Forman D., Al-Dabbagh S., Doll R. Nitrates, nitrites and gastric cancer in Great Britain. Nature. 1985 Feb 21;313(6004):620–625. doi: 10.1038/313620a0. [DOI] [PubMed] [Google Scholar]
- Fujihara S., Nakashima T., Kurogochi Y. Determination of 15NH3 by gas chromatography-mass spectrometry. Application to the measurement of putrescine oxidation by human plasma. J Chromatogr. 1986 Dec 19;383(2):271–280. [PubMed] [Google Scholar]
- Keeney D. R., Chen R. L., Graetz D. A. Importance of denitrification and nitrate reduction in sediments to the nitrogen budgets of lakes. Nature. 1971 Sep 3;233(5314):66–67. doi: 10.1038/233066a0. [DOI] [PubMed] [Google Scholar]
- Koike I., Hattori A. Denitrification and ammonia formation in anaerobic coastal sediments. Appl Environ Microbiol. 1978 Feb;35(2):278–282. doi: 10.1128/aem.35.2.278-282.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Walter B., Hollocher T. C. Respiration-dependent proton translocation and the transport of nitrate and nitrite in Paracoccus denitrificans and other denitrifying bacteria. Biochemistry. 1978 Nov 14;17(23):5014–5019. doi: 10.1021/bi00616a024. [DOI] [PubMed] [Google Scholar]
- Lombardi T. P., Gundersen B., Zammett L. O., Walters J. K., Morris B. A. Efficacy of 0.9% sodium chloride injection with or without heparin sodium for maintaining patency of intravenous catheters in children. Clin Pharm. 1988 Nov;7(11):832–836. [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks L. E., Barker H. A. THE INFLUENCE OF OXYGEN ON NITRATE AND NITRITE REDUCTION. J Bacteriol. 1949 Jul;58(1):11–22. doi: 10.1128/jb.58.1.11-22.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater J. M., Capone D. G. Denitrification in aquifer soil and nearshore marine sediments influenced by groundwater nitrate. Appl Environ Microbiol. 1987 Jun;53(6):1292–1297. doi: 10.1128/aem.53.6.1292-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S. Dissimilatory Reduction of NO(2) to NH(4) and N(2)O by a Soil Citrobacter sp. Appl Environ Microbiol. 1982 Apr;43(4):854–860. doi: 10.1128/aem.43.4.854-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Duff J. H. Denitrification in a sand and gravel aquifer. Appl Environ Microbiol. 1988 May;54(5):1071–1078. doi: 10.1128/aem.54.5.1071-1078.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje J. M., Sexstone A. J., Myrold D. D., Robinson J. A. Denitrification: ecological niches, competition and survival. Antonie Van Leeuwenhoek. 1982;48(6):569–583. doi: 10.1007/BF00399542. [DOI] [PubMed] [Google Scholar]


