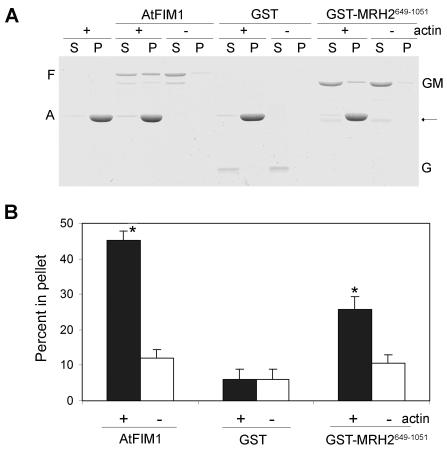
Figure 8. Binding of the ARM domain-containing MRH2649-1051 polypeptide to the polymerized actin in vitro.
(A) A high-speed co-sedimentation assay for the binding of GST-MRH2649-1051 Fragment to the polymerized actin. Equal amounts of GST-MRH2649-1051 proteins were loaded in the presence (+) or the absence (−) of the polymerized actin. After high-speed centrifugation, proteins from the supernatant (S) and the pellet (P) were separated on the SDS-PAGE gel. AtFIM1 was used as a positive control, while GST as a negative control. The position of actin (A) and AtFIM1 (F) are marked at the left, and the positions of GST (G) and GST-MRH2649-1051 (GM) are indicated at the right. Note that GST-MRH2649-1051 might have a slight degradation (bands indicated by the arrow on the right). (B) Quantitative analysis of the percentage of proteins in the pellet. The three resulting gels were scanned to determine the amount of proteins that was present in the supernatant and the pellet fractions. After the background subtraction, the percentage of proteins that co-sedimented with the polymerized actin was calculated by dividing the intensities in the pellet from the sum of that in the pellet and the supernatant. The bar represents the average and the SD of three repeated experiments. The asterisk (*) above the column indicates a significant difference (p<0.05) compared to the control of no actin.