Table 1.
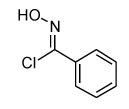
Isoxazoles formed from the 1,3-dipolar cycloaddition reactions using carbonyl containing bromoalkenes as the dipolarophile.
| Entry | Alkene | α-Chloro oxime | Product | % Yield |
|---|---|---|---|---|
| 1 | 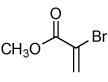 |
 |
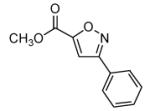 |
79 |
| 2 | 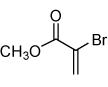 |
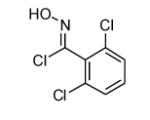 |
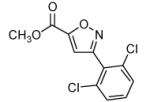 |
68 |
| 3 | 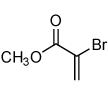 |
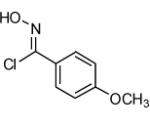 |
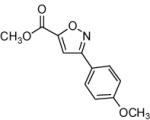 |
64 |
| 4 | 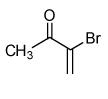 |
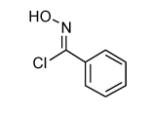 |
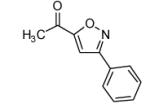 |
75 |
| 5 | 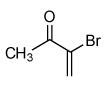 |
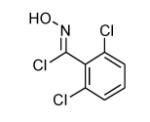 |
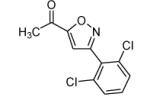 |
80 |
| 6 | 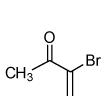 |
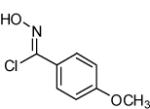 |
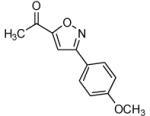 |
80 |
Isoxazoles formed from the 1,3-dipolar cycloaddition reactions using carbonyl containing bromoalkenes as the dipolarophile.
| Entry | Alkene | α-Chloro oxime | Product | % Yield |
|---|---|---|---|---|
| 1 |  |
 |
 |
79 |
| 2 |  |
 |
 |
68 |
| 3 |  |
 |
 |
64 |
| 4 |  |
 |
 |
75 |
| 5 |  |
 |
 |
80 |
| 6 |  |
 |
 |
80 |