Table 2.
Isoxazoles formed from the 1,3-dipolar cycloaddition reactions using sulfone and sulfoxide containing bromoalkenes as the dipolarophile.
| Entry | Alkene | α-Chloro oxime | Product | % Yield |
|---|---|---|---|---|
| 7 | 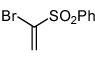 |
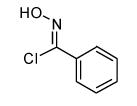 |
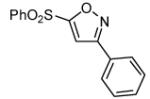 |
91 |
| 8 | 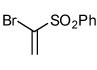 |
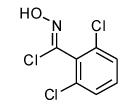 |
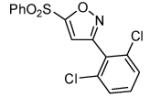 |
85 |
| 9 | 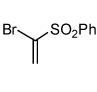 |
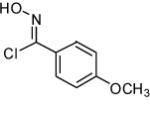 |
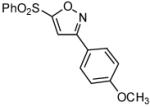 |
81 |
| 10 | 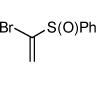 |
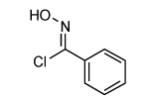 |
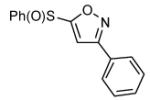 |
91 |
| 11 | 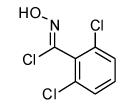 |
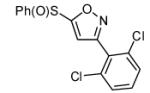 |
69 | |
| 12 | 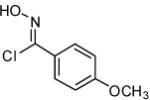 |
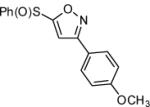 |
71 |
