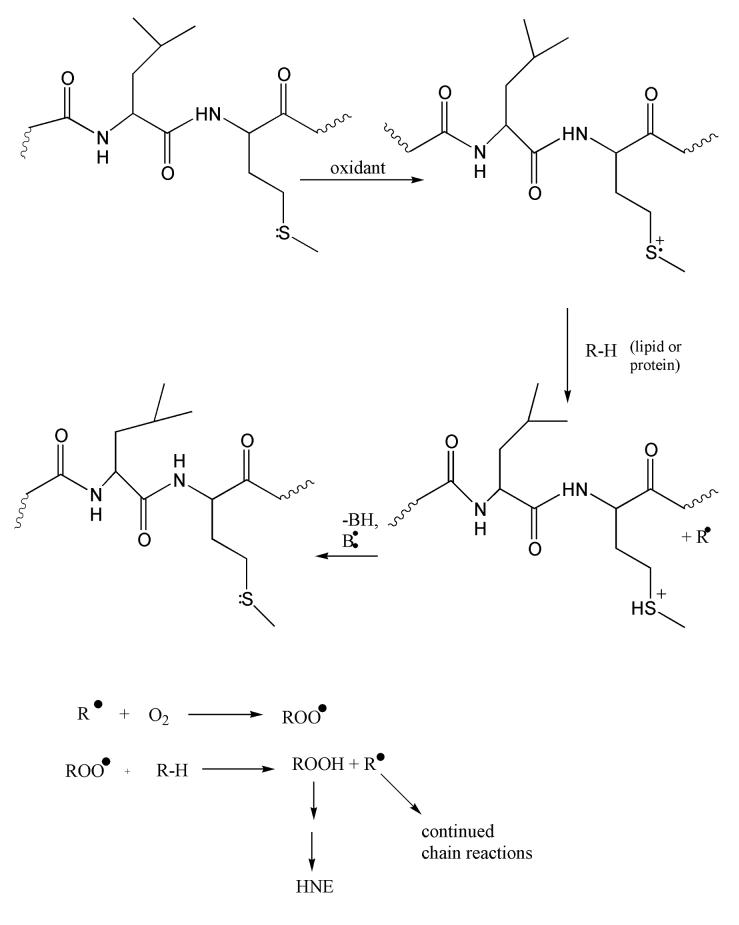
Figure 3.
Aβ(1-42) as a small oligomer is postulated to reside in the lipid bilayer. One-electron oxidation of the S-atom on Met-35, facilitated by the α-helical i + 4 interaction of the backbone carbonyl of Ile-31 with the S-atom of Met-35, forms a positively charged sulfuranyl radical that is stabilized in part by the helical dipole of Aβ(1-42) located in the lipid bilayer. This radical can abstract a lipid acyl allyllic H-atom, forming a lipid carbon radical that immediately binds paramagnetic O2. This peroxyl radical can abstract an allylic H-atom making the lipid hydroperoxide and propagating the chain reaction. The lipid hydroperoxide on arachidonic acid can decompose to HNE, which can subsequently bind to proteins by Michael addition, resulting in oxidative damage to the protein. The SH+ acid on Met has a pKa of minus 5, so loses the H+ easily to reform Met. That is, the reaction is catalytic. This mechanism is consistent with an amplification of damage by a relatively minor degree of conversion of Met to the sulfuranyl free radical of Aβ(1-42).