Figure 4.
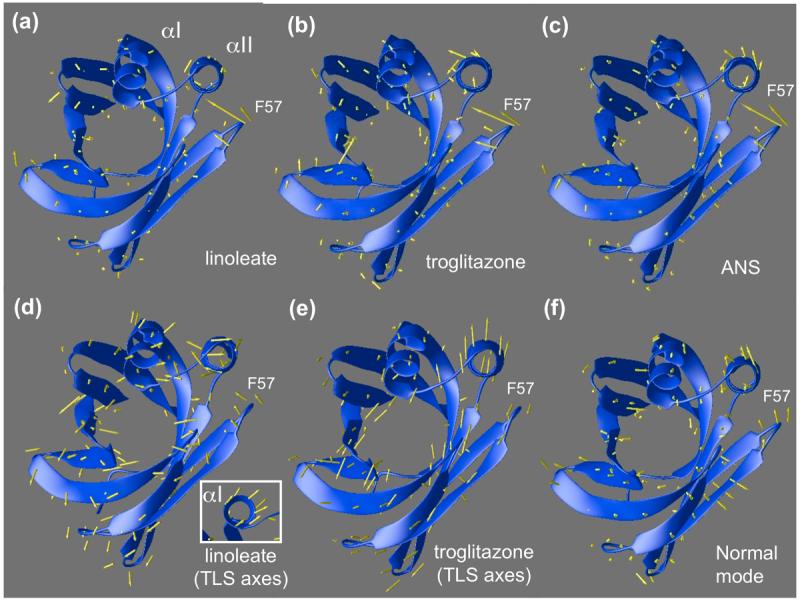
(a-c) Displacements of backbone atoms of ANS, linoleate, and troglitazone complexes relative to the non-activating oleic acid complex are shown as arrows superimposed on oleic-FABP4 ribbons. The loop containing F57 folds inward to close the binding pocket, pushing the helix αII in the same direction. (d-e) Individual atomic b-factors are isotropic in this model, but Translation, Libration, Scew (TLS) refinement allows the helix-loop-helix motif to be treated as a separate rigid unit from which aggregate anisotropic b-factors can be derived. Arrows indicate the direction of maximum anisotropy, corrected for overall movement of the protein within the lattice. (d) Linoleate complex shows movement in the F57 loop as well as helices. Helix αI shows concerted movement when viewed end-on (see inset). Linoleate appears to be more isotropic than troglitazone. (e) Troglitazone (chain A) shows systematic anisotropic movement of helix αII. The same trend is evident in chain B (not shown). (f) A linear combination of slowest vibrational modes of the (apo) backbone anticipates the helical shift induced by the ligands as well as the F57 loop movement.
