Figure 6.
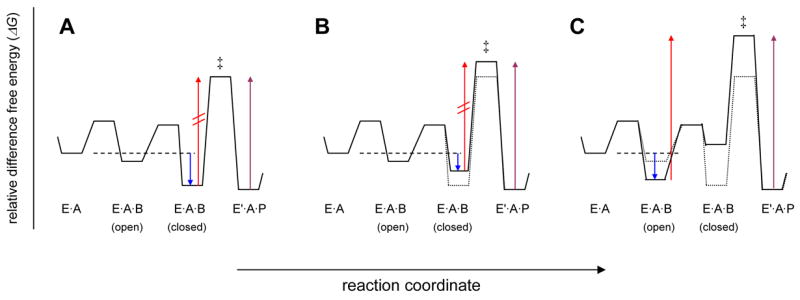
Free energy consideration of PAP. Conceptual free energy diagrams for the reaction segment, (EA + B⇌EAB(open) ⇌EAB(closed) ⇌E′AP), including a rate-limiting conversion of enzyme-bound substrates to products, are depicted. The arrows in each panel refer to free energy differences that correspond to important kinetic parameters: blue corresponds to Km (Kb); red, V1 (maximal rate in the forward direction); violet, V2 (maximal rate in reverse direction). Panel (A) describes the reaction catalyzed by wild type PAP in which the closed conformation of the enzyme substrate ternary complex is strongly favored over the open conformation. Panel (B) depicts the energetic effects of the mutation of residues involved in tertiary interactions that stabilize EAB(closed). The effect of such a mutation is to increase the energy of the EAB(closed) (ground state) and EAB‡ (transition state) by an equal amount, i.e. a uniform binding mutation. Panel (C) describes an alternative situation where the lowest-energy ground state is EAB(open), as is evidently the case for the reaction utilizing the alternative nucleotide substrate, MgCTP. The red arrow in (C) is related to the apparent maximal velocity, Vobs, where Vobs = Fc×V and Fc is the fraction of enzyme substrate complexes that are in the active, closed state.
