Abstract
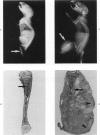
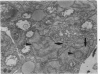
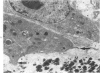
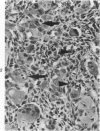
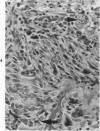
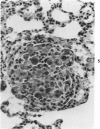
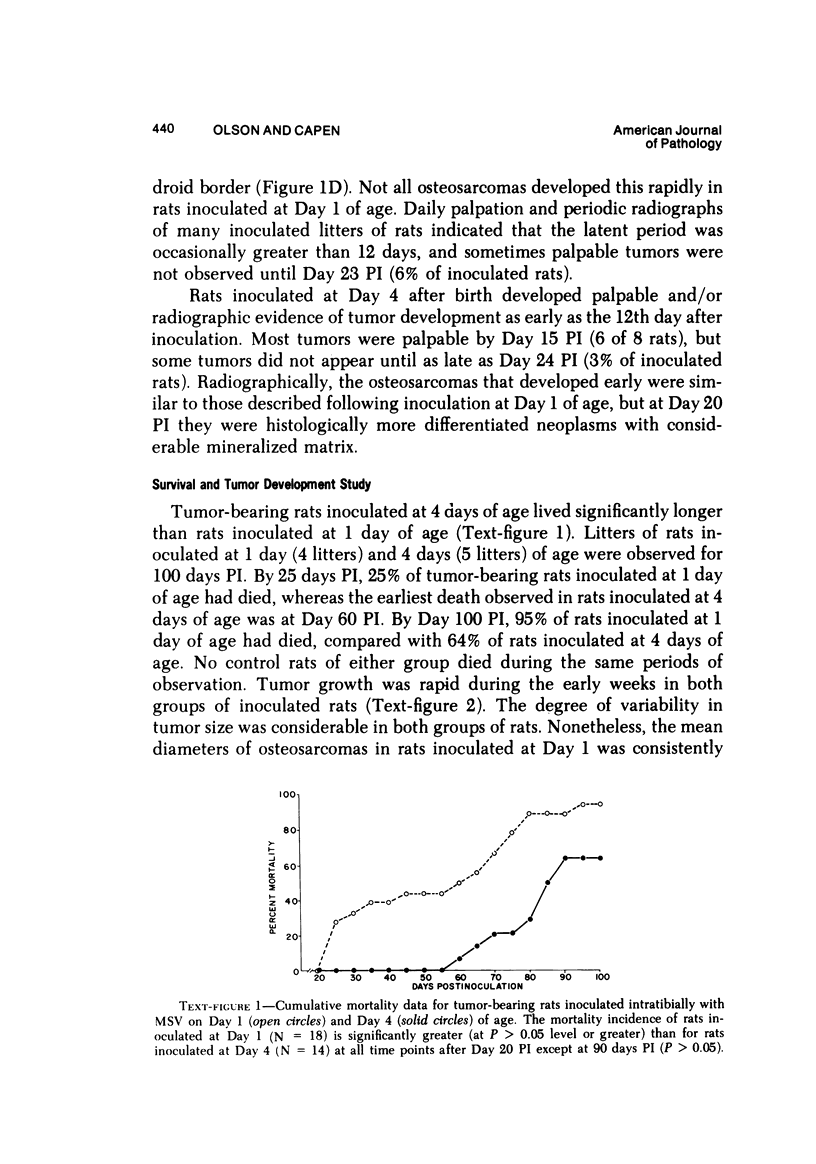
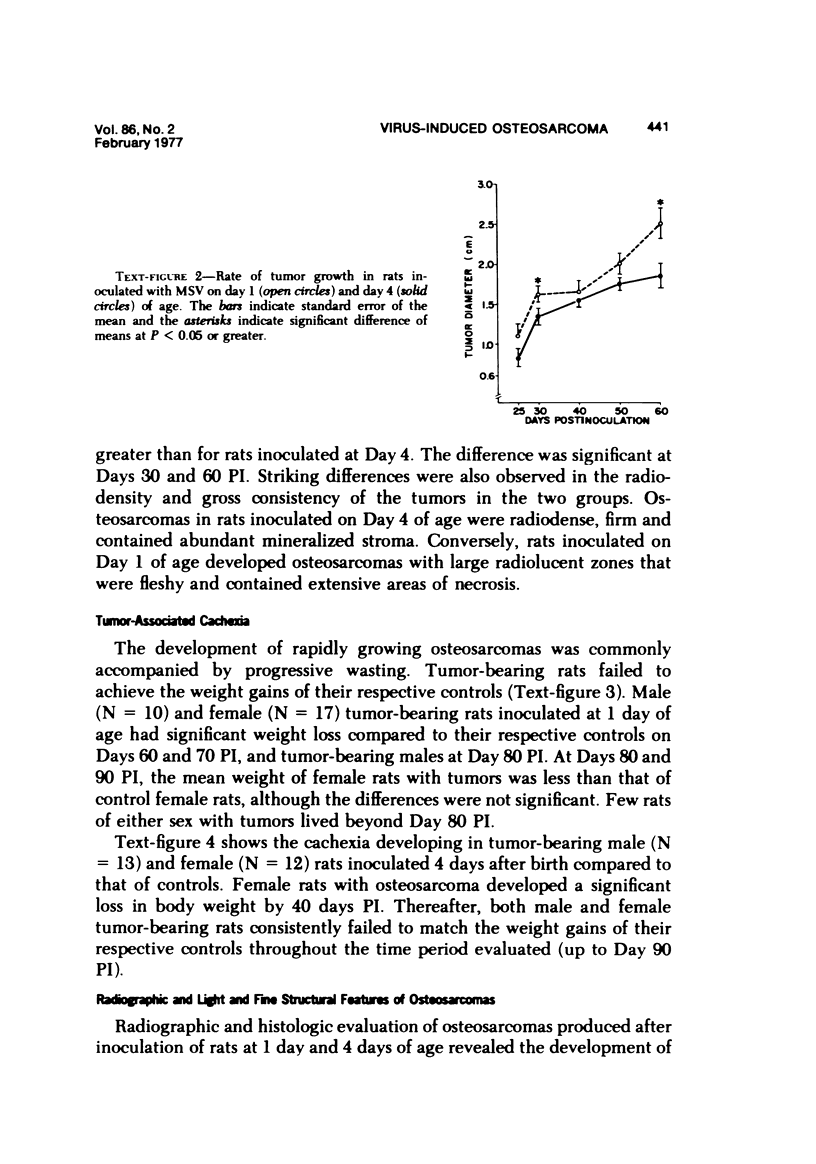
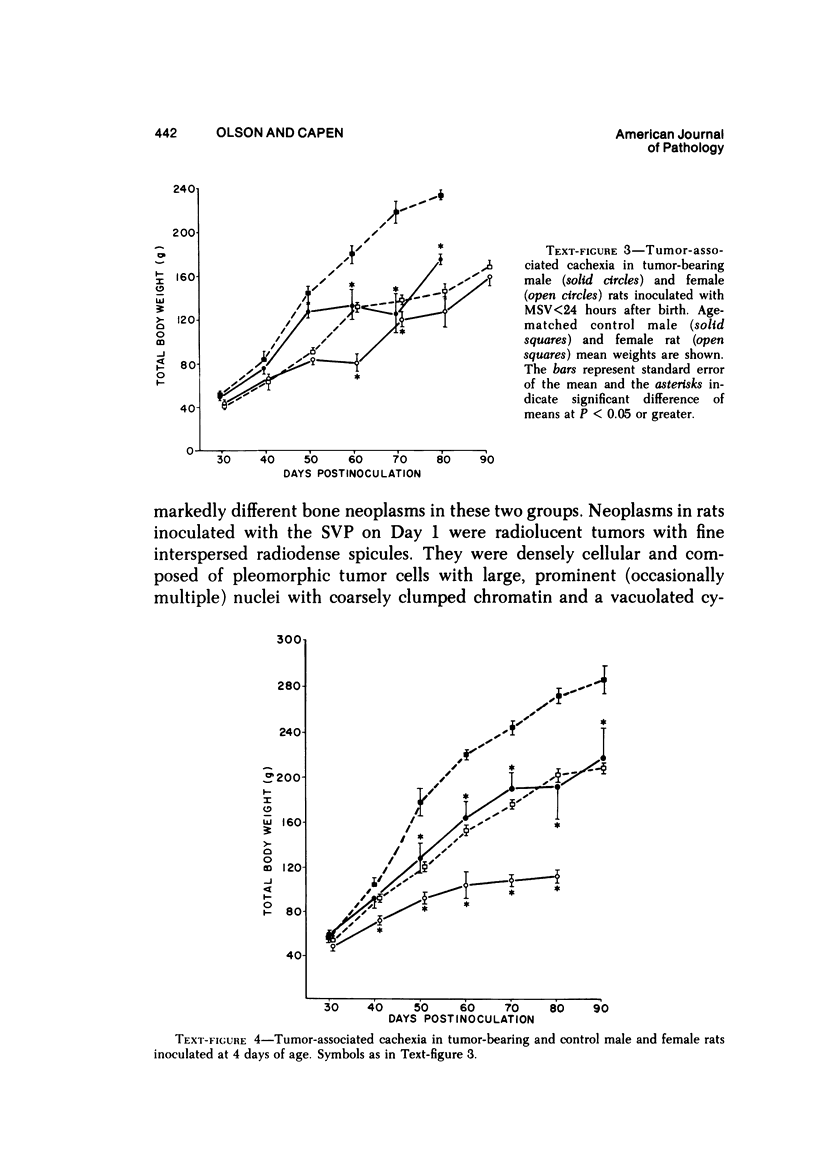
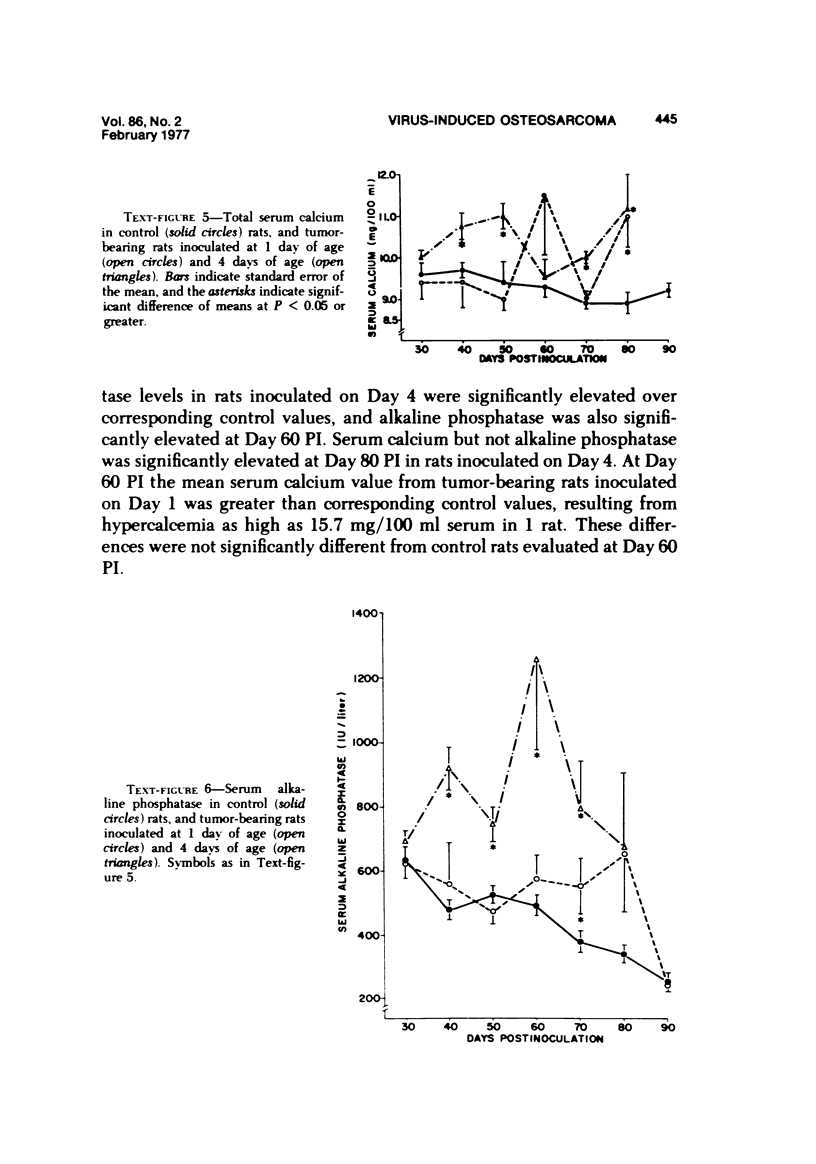
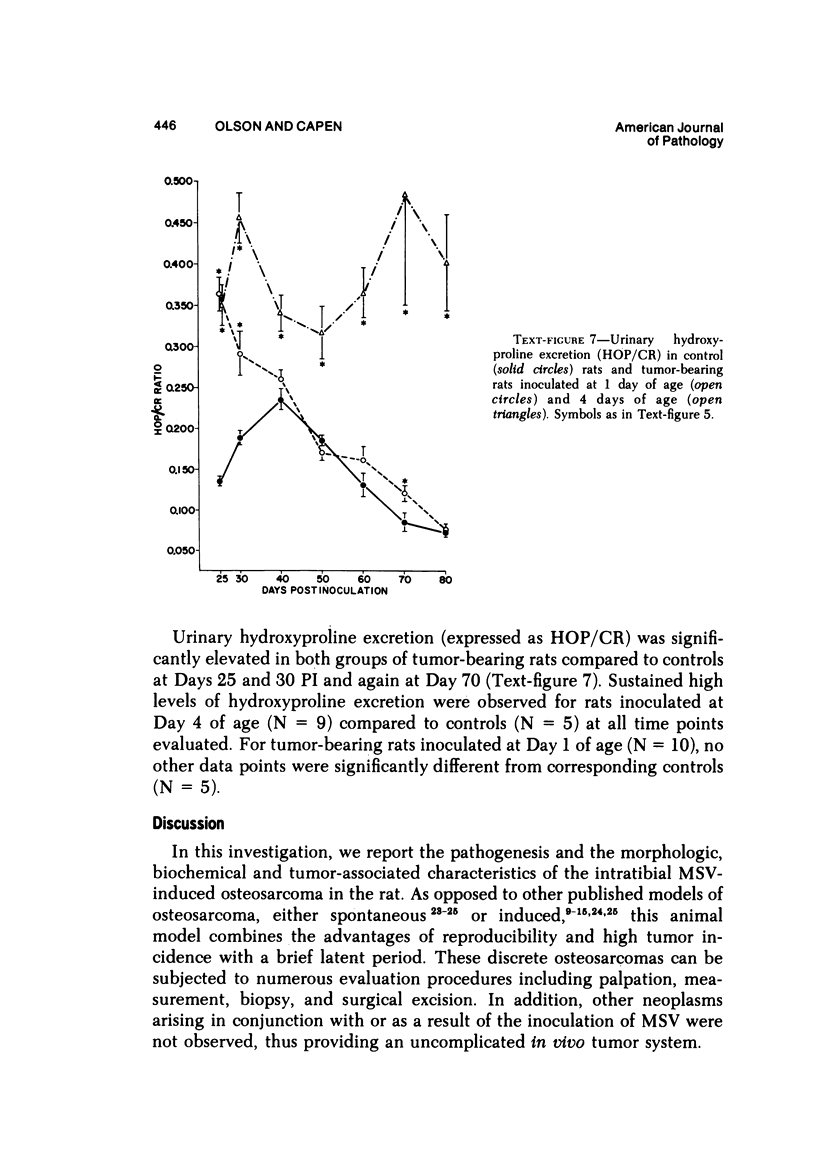
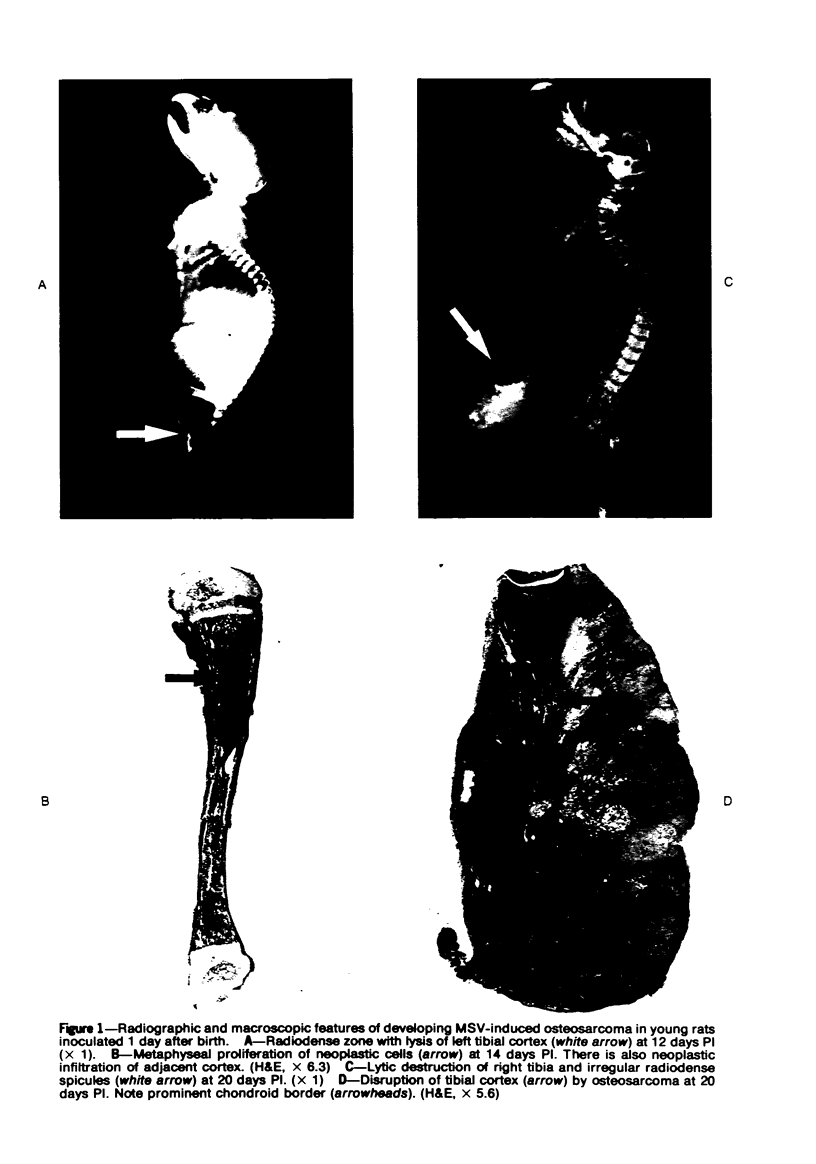
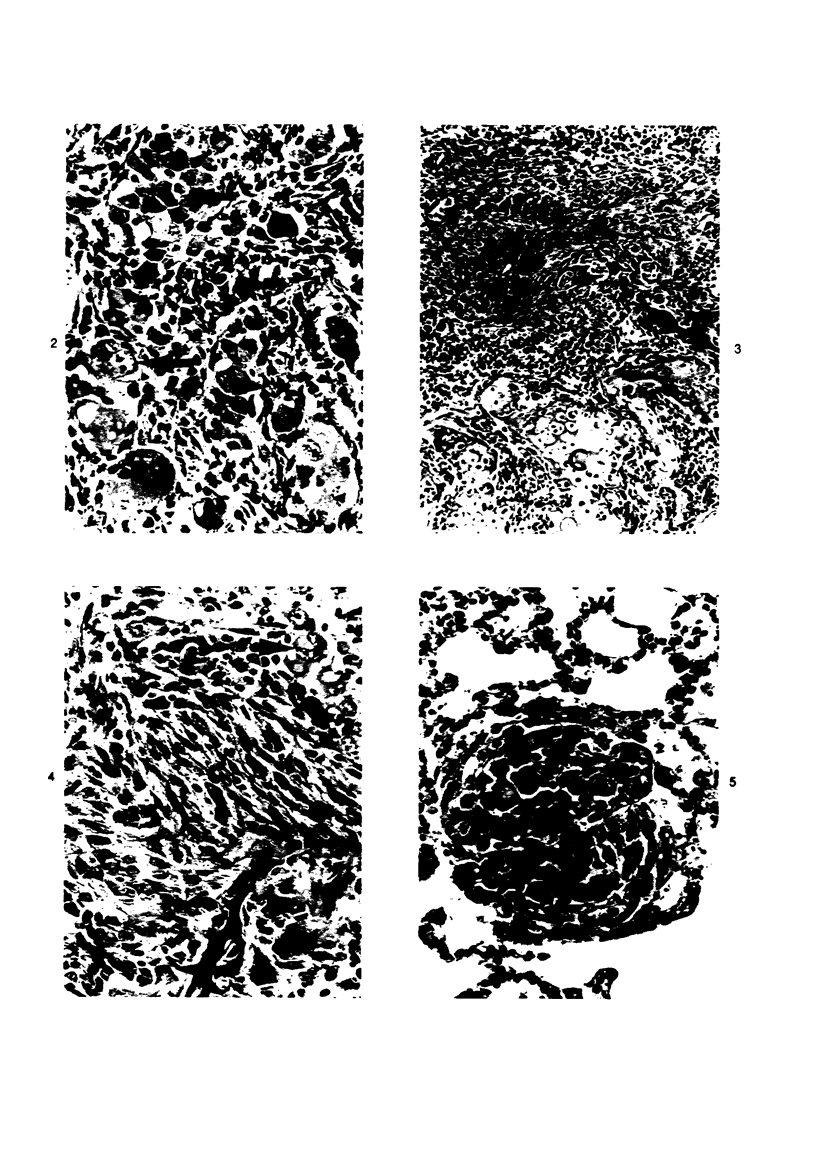
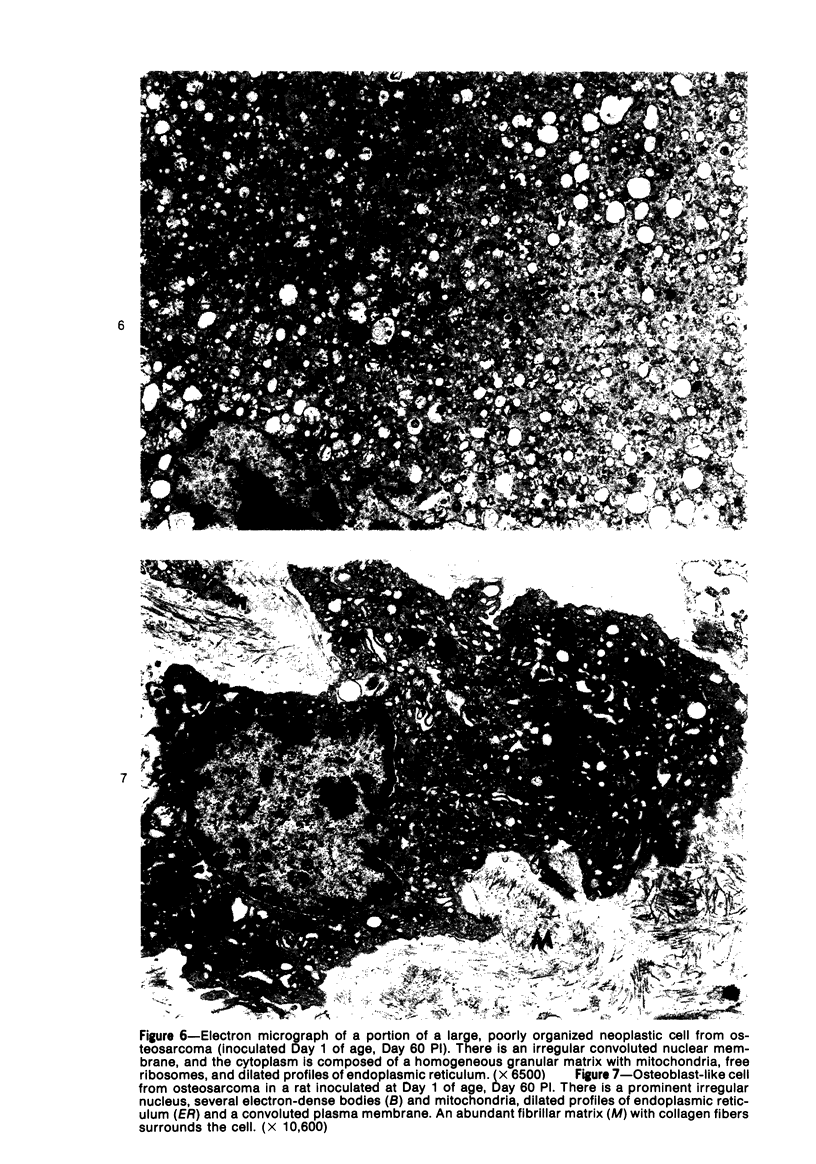
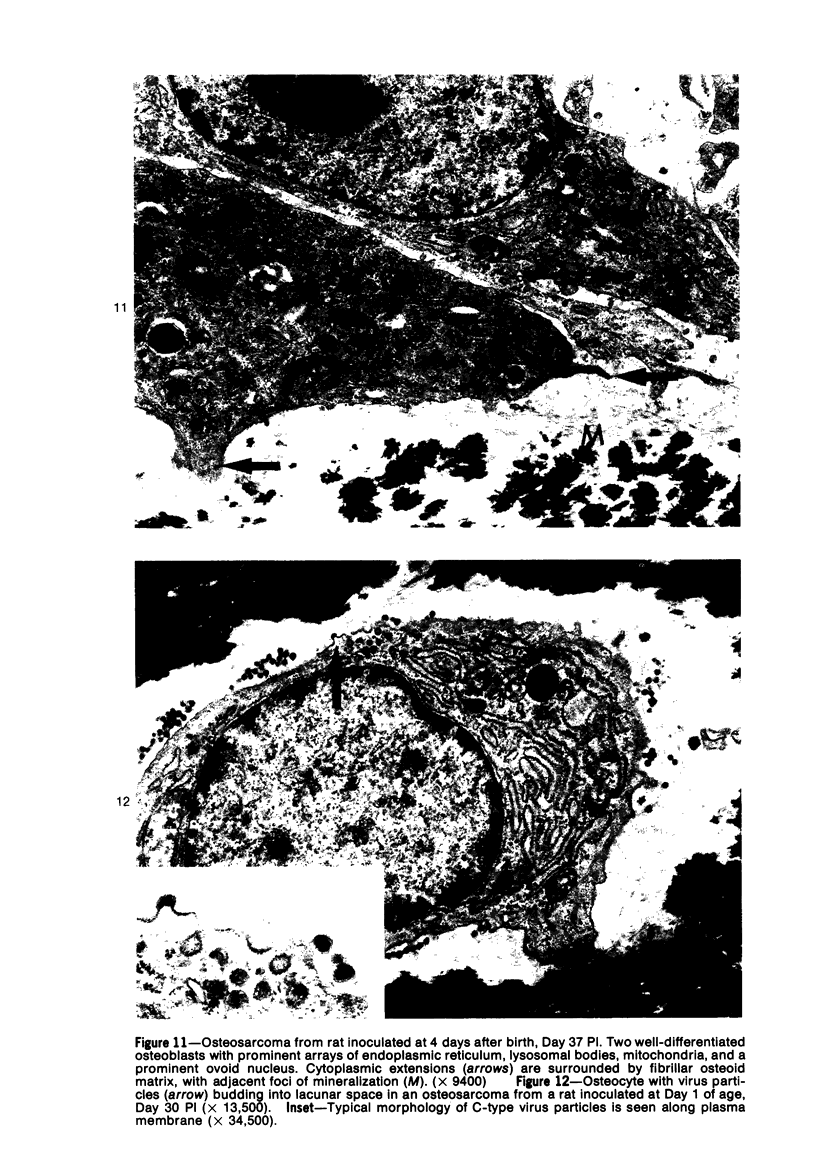
Osteosarcomas were produced by the intratibial inoculation of New Zealand black rats with Moloney sarcoma virus (MSV) at 1 day and 4 days of age. Radiographic evidence of osteosarcoma development was first demonstrated at 10 to 15 days postinoculation in both groups. Subsequent radiographic and light and electron microscopic evaluation of tumor-bearing rats demonstrated that osteosarcomas in rats inoculated at Day 4 of age were more osteoproliferative osteosarcomas than those in rats inoculated on Day 1. Rats inoculated at 4 days of age lived longer, had more slowly growing osteosarcomas, and developed a consistent tumor-associated cachexia compared to tumor-bearing rats inoculated at Day 1. Both groups of rats had a 93% metastasis rate involving either sublumbar lymph nodes, lungs, or both. Tumor-bearing rats inoculated at 4 days of age had consistent elevations in both urinary hydroxyproline excretion (HOP/CR) and serum alkaline phosphatase levels, and in serum calcium levels at some time points. The high tumor incidence after a short latent period and the morphologic and biochemical similarities between the MSV-induced murine osteosarcoma and the osteosarcoma in human beings makes this discrete tumor and a valuable animal model for the evaluation of new therapeutic regimens.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amitani K., Nakata Y. Establishment and alkaline phosphatase activity of clonal cell lines of murine osteosarcomas. A preliminary study. Clin Orthop Relat Res. 1975 Nov-Dec;(113):164–167. doi: 10.1097/00003086-197511000-00026. [DOI] [PubMed] [Google Scholar]
- Brodey R. S., Riser W. H. Canine osteosarcoma. A clinicopathologic study of 194 cases. Clin Orthop Relat Res. 1969 Jan-Feb;62:54–64. [PubMed] [Google Scholar]
- Brown G. A., Cooper R. R., Maynard J. A., Bonfiglio M. Endoplasmic reticulum size and morphology in bone disorders. Relation to protein synthesis and malignancy. Clin Orthop Relat Res. 1974 Jun;(101):278–285. [PubMed] [Google Scholar]
- CLARKE J. T. Colorimetric determination and distribution of urinary creatinine and creatine. Clin Chem. 1961 Aug;7:371–383. [PubMed] [Google Scholar]
- Cortes E. P., Holland J. F., Wang J. J., Sinks L. F., Blom J., Senn H., Bank A., Glidewell O. Amputation and adriamycin in primary osteosarcoma. N Engl J Med. 1974 Nov 7;291(19):998–1000. doi: 10.1056/NEJM197411072911903. [DOI] [PubMed] [Google Scholar]
- Diamandopoulos G. T. Induction of lymphocytic leukemia, lymphosarcoma, reticulum cell sarcoma, and osteogenic sarcoma in the Syrian golden hamster by oncogenic DNA simian virus 40. J Natl Cancer Inst. 1973 May;50(5):1347–1365. doi: 10.1093/jnci/50.5.1347. [DOI] [PubMed] [Google Scholar]
- Eilber F. R., Townsend C., Morton D. L. Osteosarcoma. Results of treatment employing adjuvant immunotherapy. Clin Orthop Relat Res. 1975 Sep;(111):94–100. [PubMed] [Google Scholar]
- Evans D. L., Barnett J. W., Dmochowski L. Immunological responsiveness in rats infected with the Soehner-Dmochowski murine sarcoma virus (MSV-SD). Tex Rep Biol Med. 1974 Summer;32(2):449–460. [PubMed] [Google Scholar]
- Finkel M. P., Biskis B. O., Jinkins P. B. Virus induction of osteosarcomas in mice. Science. 1966 Feb 11;151(3711):698–701. doi: 10.1126/science.151.3711.698. [DOI] [PubMed] [Google Scholar]
- Friedlaender G. E., Mitchell M. S. A laboratory model for the study of the immunobiology of osteosarcoma. Cancer. 1975 Nov;36(5):1631–1639. doi: 10.1002/1097-0142(197511)36:5<1631::aid-cncr2820360516>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Fujinaga S., Poel W. E., Dmochowski L. Light and electron microscope studies of osteosarcomas induced in rats and hamsters by Harvey and Moloney sarcoma viruses. Cancer Res. 1970 Jun;30(6):1698–1708. [PubMed] [Google Scholar]
- Ghadially F. N., Mehta P. N. Ultrastructure of osteogenic sarcoma. Cancer. 1970 Jun;25(6):1457–1467. doi: 10.1002/1097-0142(197006)25:6<1457::aid-cncr2820250626>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- IOACHIM H. L., CALI A., SINHA D. AGE-DEPENDENT TRANSPLANTABILITY IN RATS OF VIRUS-INDUCED THYMIC LYMPHOMA CULTURED IN VITRO. Cancer Res. 1965 Feb;25:132–139. [PubMed] [Google Scholar]
- Ikemoto K., Yamamoto T. Induction of rat osteosarcoma by inoculation of murine sarcoma virus into bone marrow. Gan. 1972 Feb;63(1):141–141. [PubMed] [Google Scholar]
- JANES J. M., HIGGINS G. M., HERRICK J. F. Beryllium-induced osteogenic sarcoma in rabbits. J Bone Joint Surg Br. 1954 Nov;36-B(4):543–552. doi: 10.1302/0301-620X.36B4.543. [DOI] [PubMed] [Google Scholar]
- Jaffe N., Frei E., 3rd, Traggis D., Bishop Y. Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med. 1974 Nov 7;291(19):994–997. doi: 10.1056/NEJM197411072911902. [DOI] [PubMed] [Google Scholar]
- Kay S. Ultrastructure of an osteoid type of osteogenic sarcoma. Cancer. 1971 Aug;28(2):437–445. doi: 10.1002/1097-0142(197108)28:2<437::aid-cncr2820280224>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- McMaster J. H., Scranton P. E., Jr, Drash A. L. Growth and hormone control mechanisms in osteosarcoma. Evidence for a new therapeutic approach. Clin Orthop Relat Res. 1975 Jan-Feb;(106):366–376. doi: 10.1097/00003086-197501000-00049. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Malmgren R. A. Human osteosarcomas: immunologic evidence suggesting an associated infectious agent. Science. 1968 Dec 13;162(3859):1279–1281. doi: 10.1126/science.162.3859.1279. [DOI] [PubMed] [Google Scholar]
- Owen L. N. Transplantation of canine osteosarcoma. Eur J Cancer. 1969 Dec;5(6):615–620. doi: 10.1016/0014-2964(69)90010-3. [DOI] [PubMed] [Google Scholar]
- Pelfrene A., Mirvish S. S., Gold B. Induction of malignant bone tumors in rats by 1-(2-hydroxyethyl)-1-nitrosourea. J Natl Cancer Inst. 1976 Feb;56(2):445–446. doi: 10.1093/jnci/56.2.445. [DOI] [PubMed] [Google Scholar]
- Pool R. R., Wolf H. G. An unusual case of canine osteosarcoma. Cancer. 1974 Sep;34(3):771–779. doi: 10.1002/1097-0142(197409)34:3<771::aid-cncr2820340337>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Pritchard D. J., Reilly C. A., Jr, Finkel M. P. Evidence for a human osteosarcoma virus. Nat New Biol. 1971 Nov 24;234(47):126–127. doi: 10.1038/newbio234126a0. [DOI] [PubMed] [Google Scholar]
- Radom S., Zulawski M., Golebiowska D. Urinary hydroxyproline, serum alkaline phosphatase, calcium and phosphorus in patients with bone neoplasms. Pol Med J. 1972;11(4):809–814. [PubMed] [Google Scholar]
- Soehner R. L., Dmochowski L. Induction of bone tumours in rats and hamsters with murine sarcoma virus and their cell-free transmission. Nature. 1969 Oct 11;224(5215):191–192. doi: 10.1038/224191a0. [DOI] [PubMed] [Google Scholar]
- Stein J. J. Osteogenic sarcoma (osteosarcoma): results of therapy. Am J Roentgenol Radium Ther Nucl Med. 1975 Mar;123(3):607–613. doi: 10.2214/ajr.123.3.607. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Editorial: Tumor humors and the hypercalcemias of cancer. N Engl J Med. 1974 Apr 18;290(16):905–906. doi: 10.1056/NEJM197404182901611. [DOI] [PubMed] [Google Scholar]
- Theologides A. Pathogenesis of cachexia in cancer. A review and a hypothesis. Cancer. 1972 Feb;29(2):484–488. doi: 10.1002/1097-0142(197202)29:2<484::aid-cncr2820290238>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Thurman G. B., Mays C. W., Taylor G. N., Keane A. T., Sissons H. A. Skeletal location of radiation-induced and naturally occurring osteosarcomas in man and dog. Cancer Res. 1973 Jul;33(7):1604–1607. [PubMed] [Google Scholar]
- Weiss P. H., Klein L. The quantitative relationship of urinary peptide hydroxyproline excretion to collagen degradation. J Clin Invest. 1969 Jan;48(1):1–10. doi: 10.1172/JCI105957. [DOI] [PMC free article] [PubMed] [Google Scholar]