Abstract
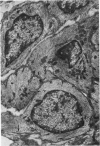
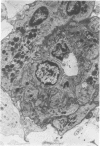
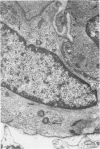
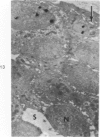
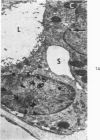
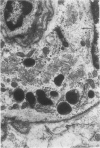
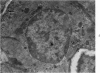
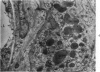
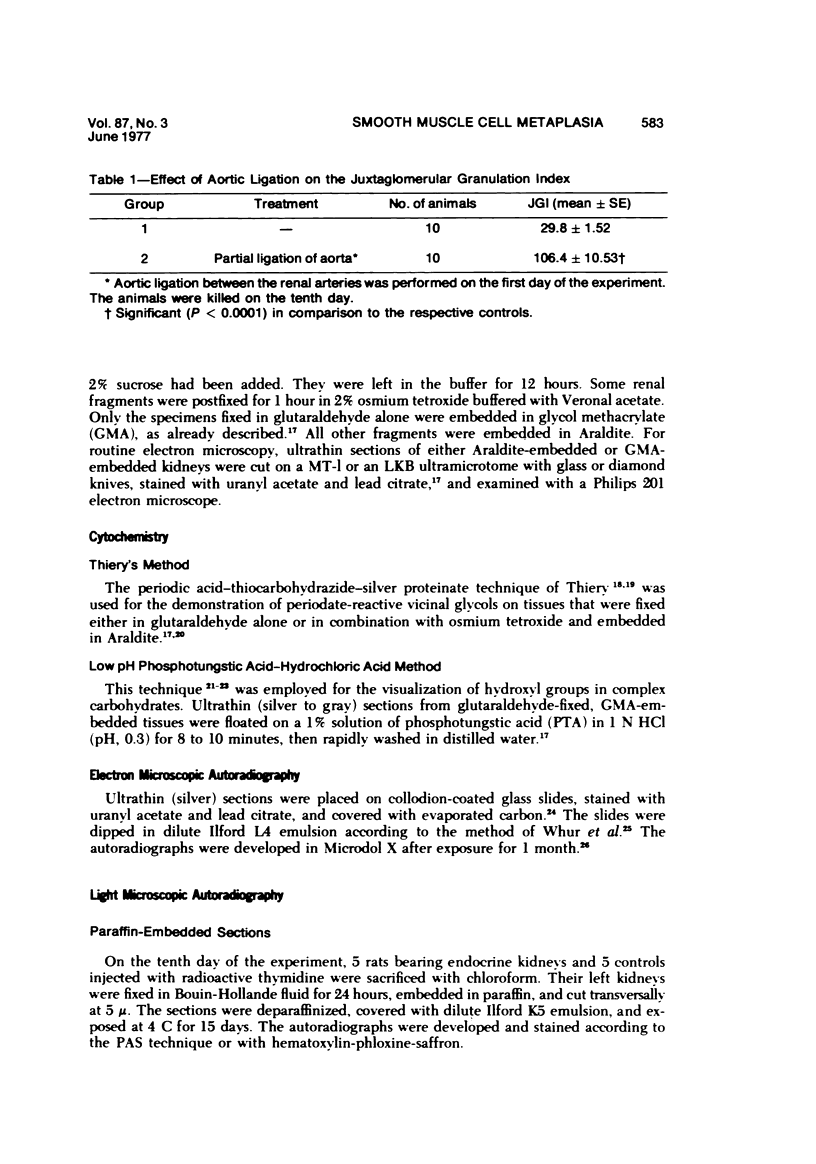
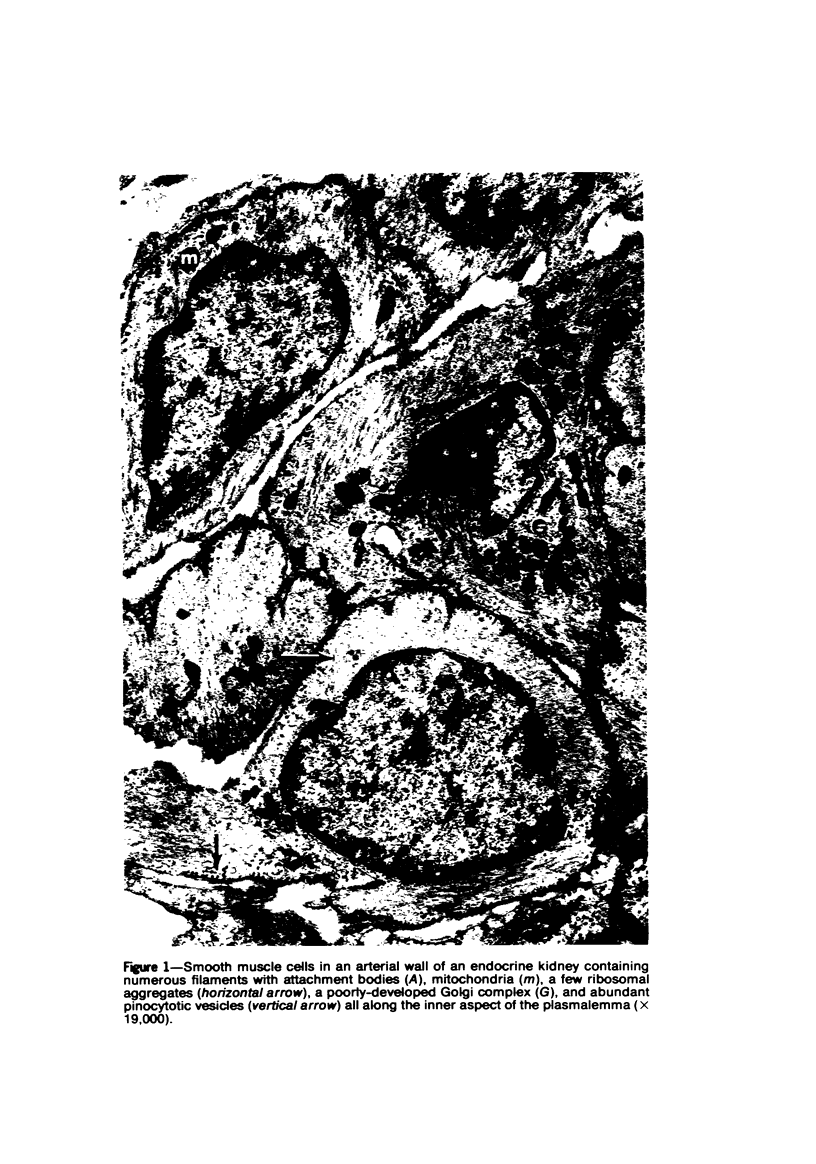
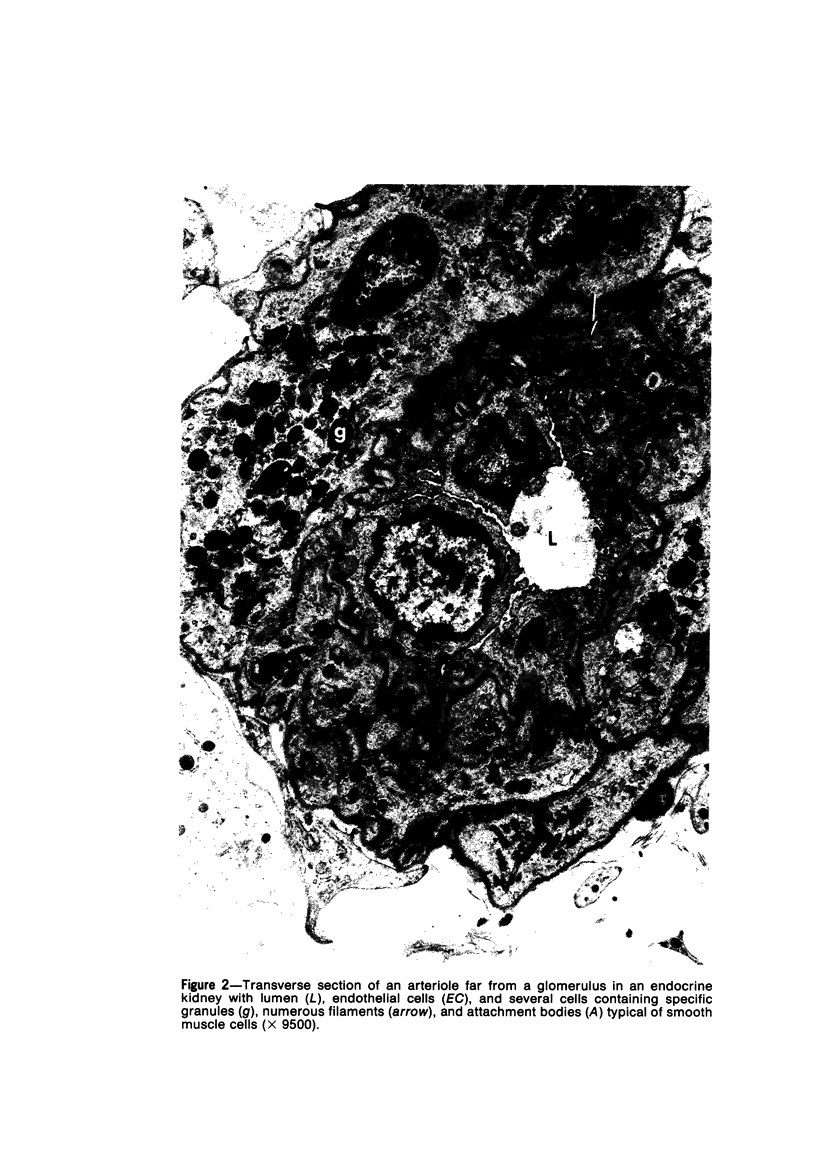
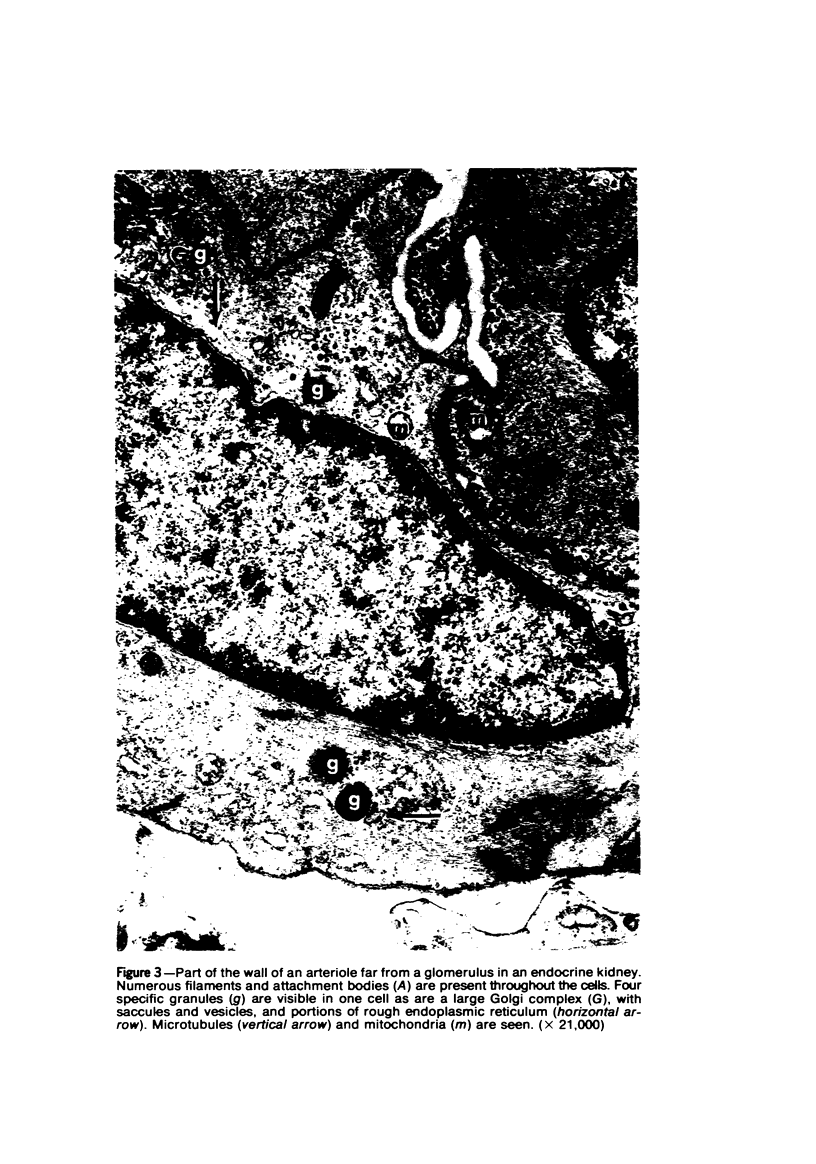
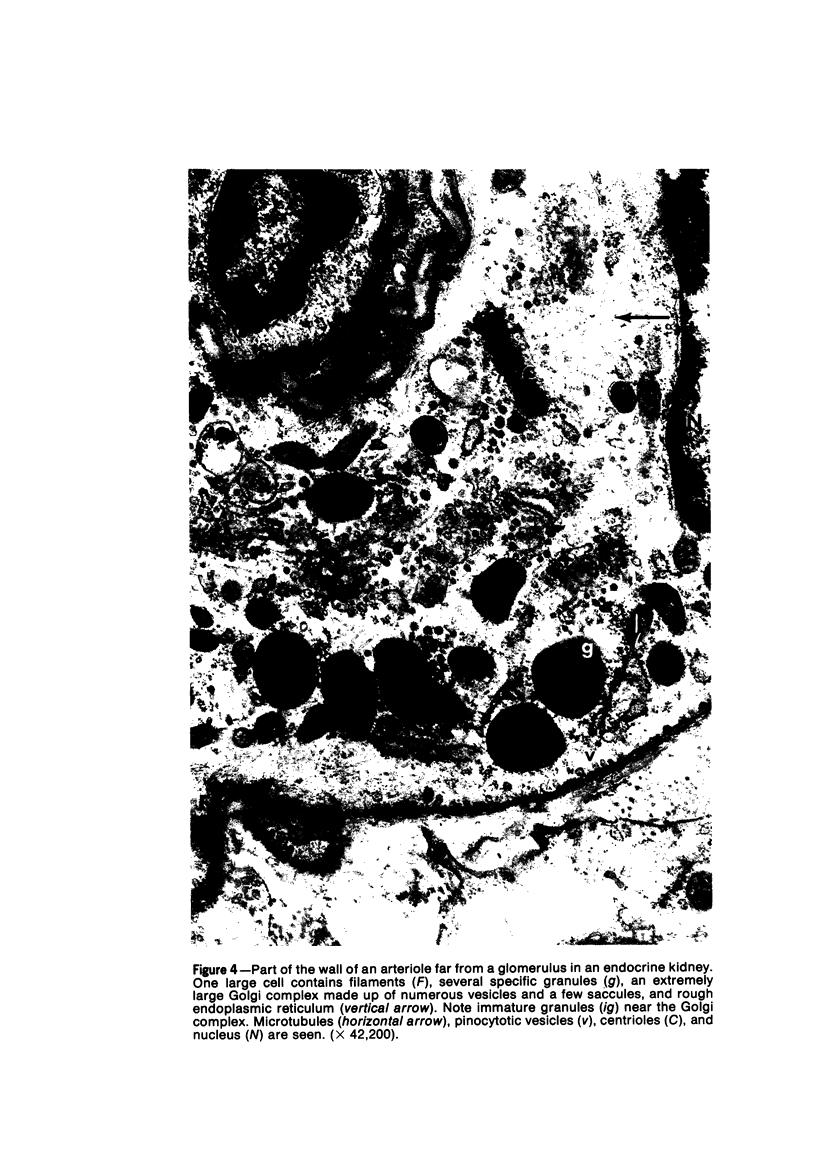
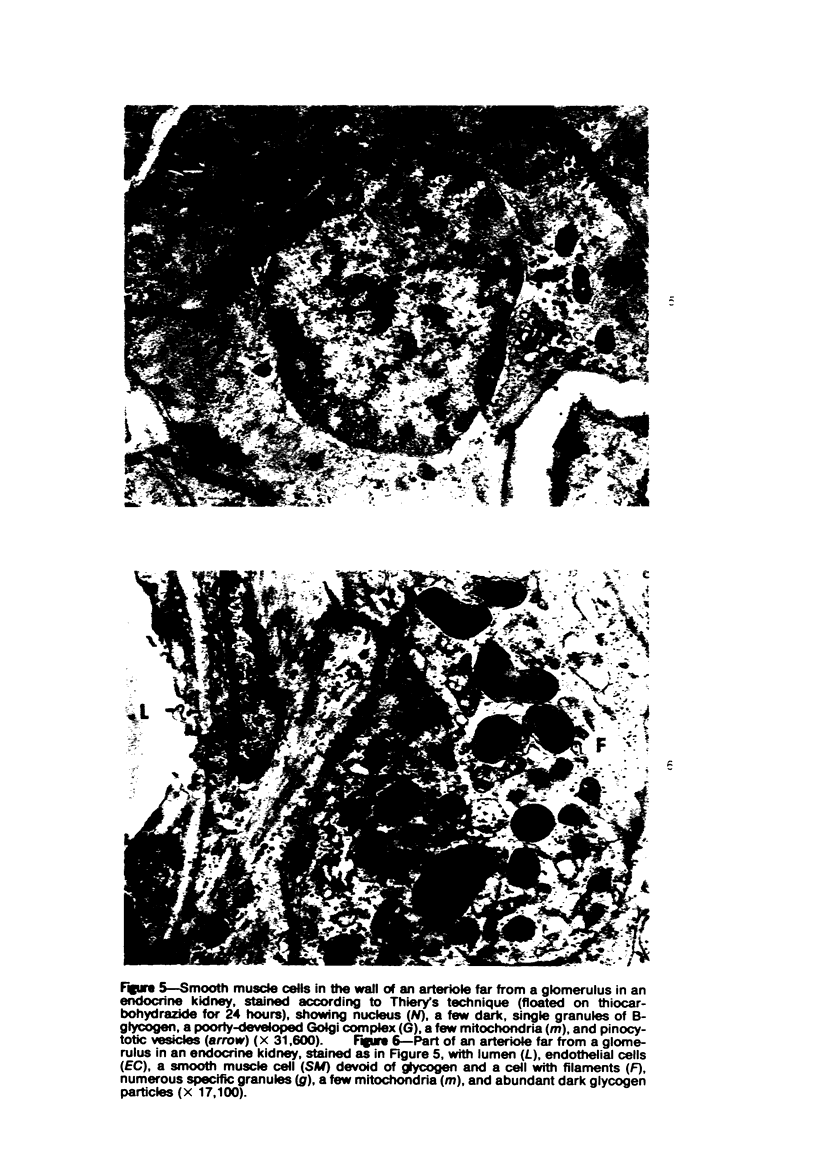
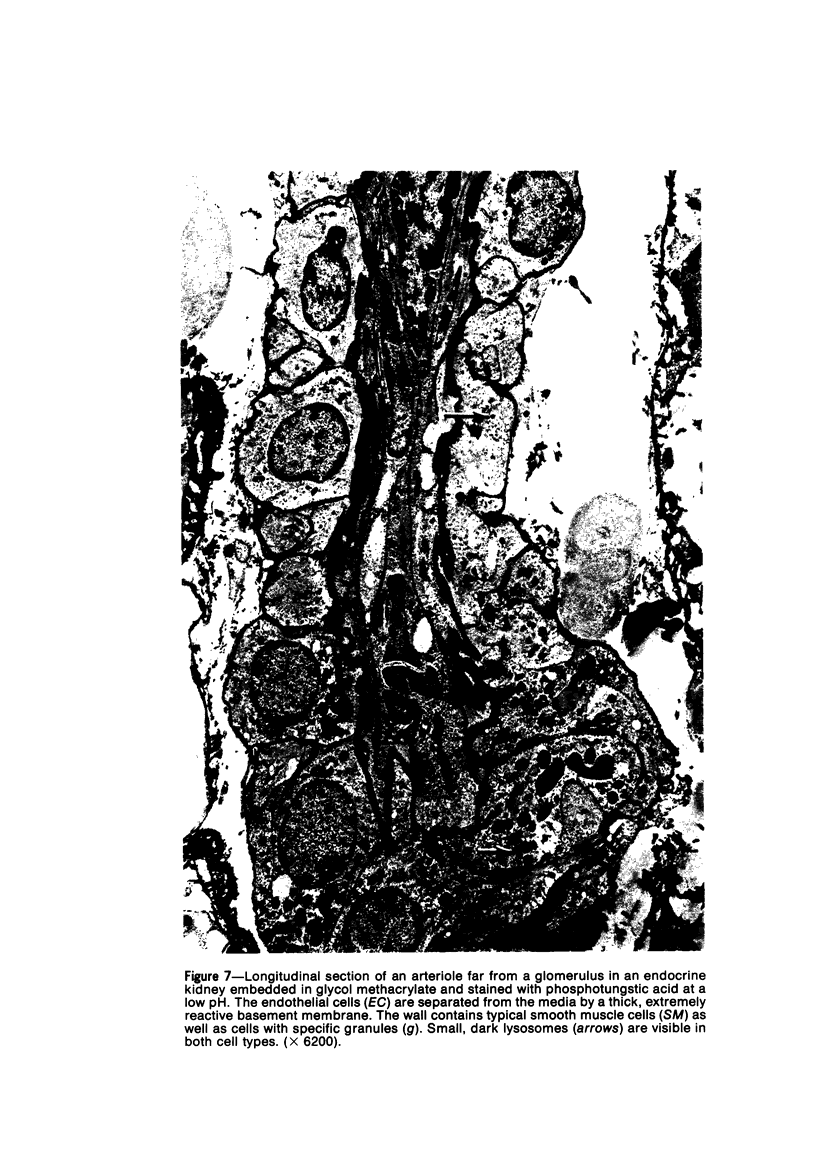
Partial ligation of the aorta between the renal arteries induces marked atrophy of the cortical tubules of the left (endocrine) kidney with a remarkable increase in the number and granularity of hypersecretory juxtaglomerular cells (JGC), which are found not only at the glomerular pole of arterioles but also in the walls of arteries and arterioles far removed from the glomerulus. Typical vascular smooth muscle cells (SMC), in which secretory granules appear, show a concomitant development of their Golgi complex and rough endoplasmic reticulum, with a gradual decrease in the number of their filaments. Microtubules also appear in the Golgi area. Thiery's periodic acid-thiocarbohydrazide-silver proteinate technique demonstrates that in these “intermediate” cells, as in mature JGC, the amount of glycogen is greater than in SMC. The newly-developed secretory granules of intermediate cells are stained by phosphotungstic acid at a low pH, as are the mature granules of JGC, an indication that both types contain glycoproteins. Light and electron microscopic autoradiography reveal that both JGC and “intermediate” cells of the vascular wall do not incorporate radioactive thymidine (injected during the 10-day observation period). Thus, they develop by metaplasia of preexistent SMC. In control kidneys, radioactive thymidine is practically never incorporated into the nuclei of SMC but is found in a few glomerular and tubular cells of all zones except the papilla.The endocrine kidney shows virtually no reactive nuclei in vascular SMC, glomeruli, or tubular cells of the outer cortex. Thymidine is incorporated into practically all nuclei of the straight portion of proximal tubules and into about half the nuclei of all medullary tubular cells including the papilla.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADEBAHR G. [Contribution to the morphology of afferent and efferent vessels of the juxtamedullary glomeruli of the human kidney]. Z Mikrosk Anat Forsch. 1962;68:48–60. [PubMed] [Google Scholar]
- Araujo-Nascimento M. d., Désormeaux Y., Cantin M. Ultrastructural cytochemistry of the ischemic (endocrine) kidney. Am J Pathol. 1976 Mar;82(3):527–548. [PMC free article] [PubMed] [Google Scholar]
- BARAJAS L., LATTA H. A three-dimensional study of the juxtaglomerular apparatus in the rat. Light and electron microscopic observations. Lab Invest. 1963 Mar;12:257–269. [PubMed] [Google Scholar]
- BENITEZ L., SHAKA J. A. CELL PROLIFERATION IN EXPERIMENTAL HYDRONEPHROSIS AND COMPENSATORY RENAL HYPERPLASIA. Am J Pathol. 1964 Jun;44:961–972. [PMC free article] [PubMed] [Google Scholar]
- BURTON A. C. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev. 1954 Oct;34(4):619–642. doi: 10.1152/physrev.1954.34.4.619. [DOI] [PubMed] [Google Scholar]
- CAMERON I. L., CLEFFMANN G. INITIATION OF MITOSIS IN RELATION TO THE CELL CYCLE FOLLOWING FEEDING OF STARVED CHICKENS. J Cell Biol. 1964 May;21:169–174. doi: 10.1083/jcb.21.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron I. L. Cell renewal in the organs and tissues of the nongrowing adult mouse. Tex Rep Biol Med. 1970 Fall;28(3):203–248. [PubMed] [Google Scholar]
- Cantin M., Benchimol S., Castonguay Y., Berlinguet J. C., Huet M. Ultrastructural cytochemistry of atrial muscle cells. V. Characterization of specific granules in the human left atrium. J Ultrastruct Res. 1975 Aug;52(2):179–192. doi: 10.1016/s0022-5320(75)80110-9. [DOI] [PubMed] [Google Scholar]
- Cantin M., Benchimol S. Localization and characterization of carbohydrates in adrenal medullary cells. J Cell Biol. 1975 May;65(2):463–469. doi: 10.1083/jcb.65.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin M., Desormeaux Y., Chlebovicova J., Benchimol S., de Araujo-Nascimento M. Comparative ultrastructural cytochemistry of juxtaglomerular cell granules and renal tubular cell lysosomes. Lab Invest. 1975 Dec;33(6):648–657. [PubMed] [Google Scholar]
- Cantin M. Relationship of juxtaglomerular apparatus and adrenal cortex to biochemical and extracellular fluid volume changes in magnesium deficiency. Lab Invest. 1970 Jun;22(6):558–568. [PubMed] [Google Scholar]
- Chopra D. P., Simnett J. D. Demonstration of an organ-specific mitotic inhibitor in amphibian kidney. The effects of adult Xenopus tissue extracts on the mitotic rate of embryonic tissue (in vitro). Exp Cell Res. 1969 Dec;58(2):319–322. doi: 10.1016/0014-4827(69)90511-4. [DOI] [PubMed] [Google Scholar]
- Chopra D. P., Simnett J. D. Stimulation of mitosis in amphibian kidney by organ specific antiserum. Nature. 1970 Feb 14;225(5233):657–658. doi: 10.1038/225657a0. [DOI] [PubMed] [Google Scholar]
- Chopra D. P., Simnett J. D. Tissue-specific mitotic inhibition in the kidneys of embryonic grafts and partially nephrectomized host Xenopus laevis. J Embryol Exp Morphol. 1971 Jun;25(3):321–329. [PubMed] [Google Scholar]
- Cuppage F. E., Cunningham N., Tate A. Nucleic acid synthesis in the regenerating nephron following injury with mercuric chloride. Lab Invest. 1969 Nov;21(5):449–457. [PubMed] [Google Scholar]
- DUNIHUE F. W., BOLDOSSER W. G. OBSERVATIONS ON THE SIMILARITY OF MESANGIAL TO JUXTAGLOMERULAR CELLS. Lab Invest. 1963 Dec;12:1228–1240. [PubMed] [Google Scholar]
- Dicker S. E. Inhibition of compensatory renal growth in rats. J Physiol. 1972 Sep;225(3):577–588. doi: 10.1113/jphysiol.1972.sp009957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker S. E., Morris C. A. Investigation of a substance of renal origin which inhibits the growth of renal cortex explant in vitro. J Embryol Exp Morphol. 1974 Jun;31(3):655–665. [PubMed] [Google Scholar]
- Dicker S. E., Shirley D. G. Compensatory hypertrophy of the contralateral kidney after unilateral ureteral ligation. J Physiol. 1972 Jan;220(1):199–210. doi: 10.1113/jphysiol.1972.sp009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., Long J. A., Jones A. L. The ultrastructure of endocrine glands. Recent Prog Horm Res. 1969;25:315–380. doi: 10.1016/b978-0-12-571125-8.50010-7. [DOI] [PubMed] [Google Scholar]
- GANDINI E., GARTLER S. M. A YEAST MITOGENIC FACTOR ACTIVE ON HUMAN PERIPHERAL LEUCOCYTES IN CULTURE. Nature. 1964 Aug 22;203:898–899. doi: 10.1038/203898b0. [DOI] [PubMed] [Google Scholar]
- GOSS R. J., RANKIN M. Physiological factors affecting compensatory renal hyperplasia in the rat. J Exp Zool. 1960 Dec;145:209–216. doi: 10.1002/jez.1401450304. [DOI] [PubMed] [Google Scholar]
- HARTROFT P. M., HARTROFT W. S. Studies on renal juxtaglomerular cells. I. Variations produced by sodium chloride and desoxycorticosterone acetate. J Exp Med. 1953 Mar;97(3):415–429. doi: 10.1084/jem.97.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATT P. Y., DONTCHEFF A. Contribution de la microscopie électronique à l'étude du mécanisme de l'hypertension artérielle expérimentale d'origine rénale chez le rat; les lésions des artères du rein. Arch Mal Coeur Vaiss. 1959 May;52(5):490–503. [PubMed] [Google Scholar]
- HATT P. Y., DVOJAKOVIC M., CORNET P. [Contribution of electron microscopy to the study of the mechanism of experimental arterial hypertension of renal origin. II. Renal ischemia in the rabbit]. Pathol Biol. 1962 Jan;10:23–40. [PubMed] [Google Scholar]
- HATT P. Y. [Secretory activity of the wall of the renal arterioles. Cytological demonstration in experimental renal ischemia]. C R Hebd Seances Acad Sci. 1961 Mar 20;252:1851–1853. [PubMed] [Google Scholar]
- Heine W. D., Stöcker E. Regeneration of kidney parenchyma under normal and pathological conditions. Beitr Pathol. 1972;145(1):89–99. [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. The juxtaglomerular apparatus as studied electron microscopically. J Ultrastruct Res. 1962 Jun;6:547–561. doi: 10.1016/s0022-5320(62)80009-4. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., MESSIER B., KOPRIWA B. Thymidine-H3 as a tool for the investigation of the renewal of cell populations. Lab Invest. 1959 Jan-Feb;8(1):296–308. [PubMed] [Google Scholar]
- Leung D. Y., Glagov S., Mathews M. B. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976 Feb 6;191(4226):475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- Lyons H. J., Evan A. P., McLaren L. C., Solomon S. In vitro evidence for a renotrophic factor in renal compensatory hypertrophy. Nephron. 1974;13(3):198–211. doi: 10.1159/000180394. [DOI] [PubMed] [Google Scholar]
- MASSON G. M., KASHII C., MATSUNAGA M., PAGE I. H. HYPERTENSIVE VASCULAR DISEASE PRODUCED BY HOMOLOGOUS RENIN. Science. 1964 Jul 10;145(3628):178–180. doi: 10.1126/science.145.3628.178. [DOI] [PubMed] [Google Scholar]
- Mandalenakis N., Cantin M., Dumont A. L'appareil juxtaglomérulaire du rein endocrinien. Etude histologique et ultrastructurale. Pathol Biol (Paris) 1970 Mar;18(5):233–240. [PubMed] [Google Scholar]
- Nagle R. B., Johnson M. E., Jervis H. R. Proliferation of renal interstitial cells following injury induced by ureteral obstruction. Lab Invest. 1976 Jul;35(1):18–22. [PubMed] [Google Scholar]
- Pease D. C. Polysaccharides associated with the exterior surface of epithelial cells: kidney, intestine, brain. J Ultrastruct Res. 1966 Aug;15(5):555–588. doi: 10.1016/s0022-5320(66)80128-4. [DOI] [PubMed] [Google Scholar]
- Pederson T., Gelfant S. G2-population cells in mouse kidney and duodenum and their behavior during the cell division cycle. Exp Cell Res. 1970 Jan;59(1):32–36. doi: 10.1016/0014-4827(70)90620-8. [DOI] [PubMed] [Google Scholar]
- Perry L. D., Swartz F. J. Evidence for a subpopulation of cells with an extended G2 period in normal adult mouse liver. Exp Cell Res. 1967 Oct;48(1):155–157. doi: 10.1016/0014-4827(67)90288-1. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Goldin H. A renotropic system in rats. J Clin Invest. 1976 Jan;57(1):94–101. doi: 10.1172/JCI108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss H. G., Goldin H. Humoral regulation of compensatory renal growth. Med Clin North Am. 1975 May;59(3):771–780. doi: 10.1016/s0025-7125(16)32023-5. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Terryi E. F., Keller A. I. Renotropic factor(s) in plasma from uninephrectomized rats. Nephron. 1970;7(5):459–470. doi: 10.1159/000179845. [DOI] [PubMed] [Google Scholar]
- SAETREN H. A principle of auto-regulation of growth; production of organ specific mitose-inhibitors in kidney and liver. Exp Cell Res. 1956 Aug;11(1):229–232. doi: 10.1016/0014-4827(56)90212-9. [DOI] [PubMed] [Google Scholar]
- Simnett J. D., Chopra D. P. Organ specific inhibitor of mitosis the aphibian kidney. Nature. 1969 Jun 21;222(5199):1189–1190. doi: 10.1038/2221189a0. [DOI] [PubMed] [Google Scholar]
- TURGEON C., SOMMERS S. C. Juxtaglomerular cell counts and human hypertension. Am J Pathol. 1961 Feb;38:227–241. [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS G. E. Some aspects of compensatory hyperplasia of the kidney. Br J Exp Pathol. 1961 Aug;42:386–396. [PMC free article] [PubMed] [Google Scholar]
- WOLINSKY H., GLAGOV S. STRUCTURAL BASIS FOR THE STATIC MECHANICAL PROPERTIES OF THE AORTIC MEDIA. Circ Res. 1964 May;14:400–413. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- Whur P., Herscovics A., Leblond C. P. Radioautographic visualization of the incorporation of galactose-3H and mannose-3H by rat thyroids in vitro in relation to the stages of thyroglobulin synthesis. J Cell Biol. 1969 Nov;43(2):289–311. doi: 10.1083/jcb.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res. 1970 Apr;26(4):507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]