Abstract
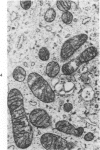
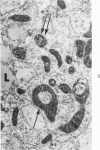
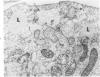
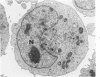
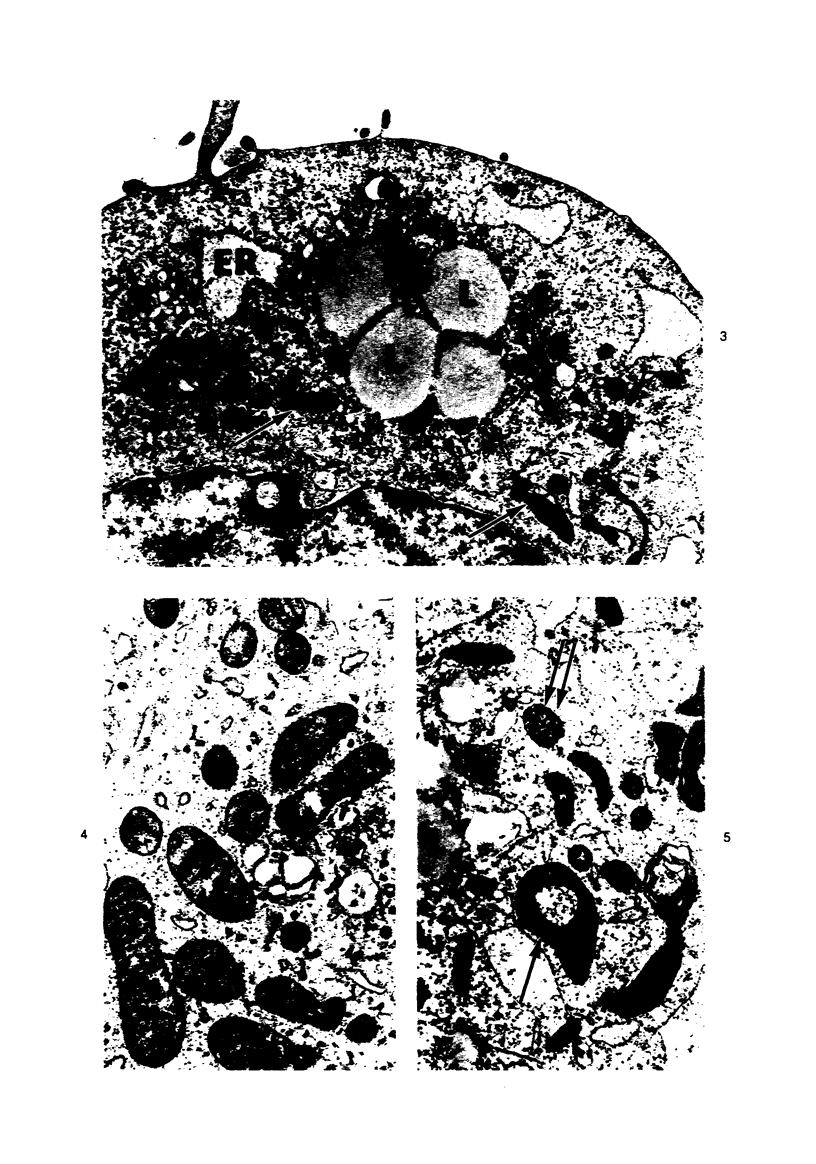
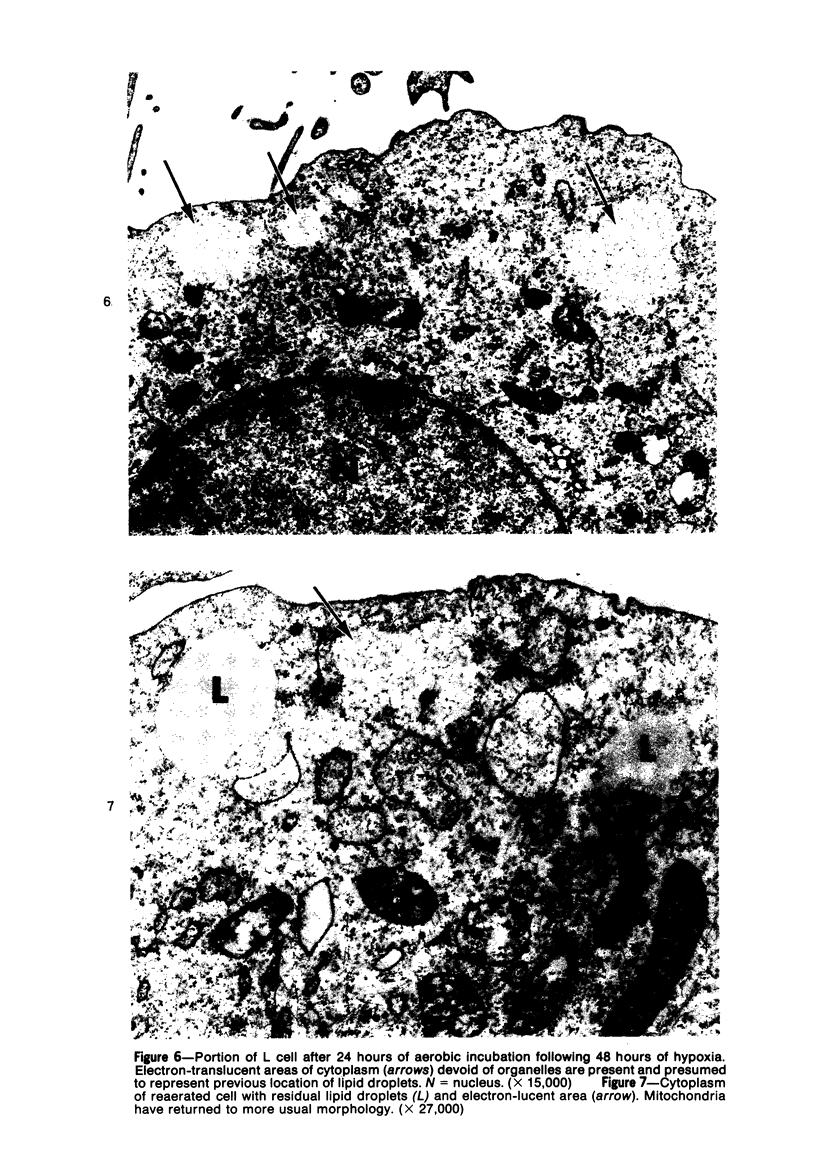
Lipid droplets have long been recognized by light microscopy to accumulate in hypoxic cells both in vivo and in vitro. In the present tissue culture experiments, correlative electron microscopic observations and lipid analyses were performed to determine the nature and significance of lipid accumulation in hypoxia. Strain L mouse fibroblasts were grown in suspension culture, both aerobically and under severe oxygen restriction obtained by gassing cultures daily with an 8% CO2-92% nitrogen mixture. After 48 hours, hypoxic cells showed an increase in total lipid/protein ratio of 42% over control cells. Most of this increase was accounted for by an elevation in the level of cellular triglyceride from 12.3 ± 0.9 μg/mg cell protein in aerobic cultures to 41.9 ± 0.7 in the hypoxic cultures, an increase of 240%. Levels of cellular free fatty acids (FFA) were 96% higher in the hypoxic cultures. No significant changes in the levels of cellular phospholipid or cholesterol were noted. Electron microscopic examination revealed the accumulation of homogeneous cytoplasmic droplets. The hypoxic changes were reversible upon transferring the cultures to aerobic atmospheres with disappearance of the lipid. These experiments indicate that hypoxic injury initially results in triglyceride and FFA accumulation from an inability to oxidize fatty acids taken up from the media and not from autophagic processes, as described in other types of cell injury associated with the sequestration of membranous residues and intracellular cholesterol and phospholipid accumulation.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADEBONOJO F. O., BENSCH K. G., KING D. W. The effect of nitrogen on the enzymatic pattern of strain L cells. Cancer Res. 1961 Feb;21:252–256. [PubMed] [Google Scholar]
- Albers J. J., Bierman E. L. The effect of hypoxia on uptake and degradation of low density lipoproteins by cultured human arterial smooth muscle cells. Biochim Biophys Acta. 1976 Mar 26;424(3):422–429. doi: 10.1016/0005-2760(76)90031-x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BENSCH K., GORDON G., MILLER L. THE FATE OF DNA-CONTAINING PARTICLES PHAGOCYTIZED BY MAMMALIAN CELLS. J Cell Biol. 1964 Apr;21:105–114. doi: 10.1083/jcb.21.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIAMORI N., HENRY R. J. Study of the ferric chloride method for determination of total cholesterol and cholesterol esters. Am J Clin Pathol. 1959 Apr;31(4):305–309. doi: 10.1093/ajcp/31.4.305. [DOI] [PubMed] [Google Scholar]
- Chiodi H. Study of possible causal mechanism of fatty liver of chronic hypoxic suckling rats. Am J Physiol. 1970 Jan;218(1):92–94. doi: 10.1152/ajplegacy.1970.218.1.92. [DOI] [PubMed] [Google Scholar]
- Constantinides P., Robinson M. Ultrastructural injury of arterial endothelium. 1. Effects of pH, osmolarity, anoxia, and temperature. Arch Pathol. 1969 Aug;88(2):99–105. [PubMed] [Google Scholar]
- DALES S., FISHER K. C. The effect of carbon monoxide on oxygen consumption, glucose utilization, and growth in mammalian cells in vitro. Can J Biochem Physiol. 1959 May;37(5):623–638. [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G., Cohn Z. A. Autophagic vacuoles produced in vitro. II. Studies on the mechanism of formation of autophagic vacuoles produced by chloroquine. J Cell Biol. 1968 Aug;38(2):392–402. doi: 10.1083/jcb.38.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. J. A colorimetric method for estimating serum triglycerides. Clin Chim Acta. 1968 Nov;22(3):393–397. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Frings C. S., Dunn R. T. A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clin Pathol. 1970 Jan;53(1):89–91. doi: 10.1093/ajcp/53.1.89. [DOI] [PubMed] [Google Scholar]
- GORDON G. B., MILLER L. R., BENSCH K. G. FIXATION OF TISSUE CULTURE CELLS FOR ULTRASTRUCTURAL CYTOCHEMISTRY. Exp Cell Res. 1963 Aug;31:440–443. doi: 10.1016/0014-4827(63)90024-7. [DOI] [PubMed] [Google Scholar]
- Geyer R. P. Uptake and retention of fatty acids by tissue culture cells. Wistar Inst Symp Monogr. 1967;6:33–47. [PubMed] [Google Scholar]
- Gordon G. B. Lipid accumulation in the stationary phase of strain L cells in suspension culture. Lab Invest. 1977 Feb;36(2):114–121. [PubMed] [Google Scholar]
- Henderson A. H., Most A. S., Parmley W. W., Gorlin R., Sonnenblick E. H. Depression of myocardial contractility in rats by free fatty acids during hypoxia. Circ Res. 1970 Apr;26(4):439–449. doi: 10.1161/01.res.26.4.439. [DOI] [PubMed] [Google Scholar]
- JENNINGS R. B., WARTMAN W. B. Reactions of the myocardium to obstruction of the coronary arteries. Med Clin North Am. 1957 Jan;41(1):3–15. doi: 10.1016/s0025-7125(16)34461-3. [DOI] [PubMed] [Google Scholar]
- KING D. W., SOCOLOW E. L., BENSCH K. G. The relation between protein synthesis and lipide accumulation in L strain cells and Ehrlich ascites cells. J Biophys Biochem Cytol. 1959 May 25;5(3):421–431. doi: 10.1083/jcb.5.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUCHLER R. J., MERCHANT D. J. Propagation of strain L (Earle) cells in agitated fluid suspension cultures. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):803–806. doi: 10.3181/00379727-92-22620. [DOI] [PubMed] [Google Scholar]
- King M. E., Godman G. C., King D. W. Respiratory enzymes and mitochondrial morphology of HeLa and L cells treated with chloramphenicol and ethidium bromide. J Cell Biol. 1972 Apr;53(1):127–142. doi: 10.1083/jcb.53.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. E., King D. W. Respiratory enzyme activity and mitochondrial morphology of L-cells under prolonged oxygen deprivation. Lab Invest. 1971 Nov;25(5):374–379. [PubMed] [Google Scholar]
- Kurien V. A., Yates P. A., Oliver M. F. The role of free fatty acids in the production of ventricular arrhythmias after acute coronary artery occlusion. Eur J Clin Invest. 1971 Jan;1(4):225–241. doi: 10.1111/eci.1971.1.4.225. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE C. G., MACKENZIE J. B., BECK P. The effect of pH on growth, protein synthesis, and lipid-rich particles of cultured mammalian cells. J Biophys Biochem Cytol. 1961 Jan;9:141–156. doi: 10.1083/jcb.9.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE C. G., MACKENZIE J. B., REISS O. K. REGULATION OF CELL LIPID METABOLISM AND ACCUMULATION. 3. THE LIPID CONTENT OF MAMMALIAN CELLS AND THE RESPONSE TO THE LIPOGENIC ACTIVITY OF RABBIT SERUM. Exp Cell Res. 1964 Dec;36:533–547. doi: 10.1016/0014-4827(64)90310-6. [DOI] [PubMed] [Google Scholar]
- Mackenzie C. G., Mackenzie J. B., Reiss O. K. Regulation of cell lipid metabolism and accumulation. V. Quantitative and structural aspects of triglyceride accumulation caused by lipogenic substances. Wistar Inst Symp Monogr. 1967;6:63–83. [PubMed] [Google Scholar]
- May J. F., Paule W. J., Rounds D. E., Blankenhorn D. H., Zemplenyi T. The induction of atherosclerotic plaque-like mounds in cultures of aortic smooth muscle cells. Virchows Arch B Cell Pathol. 1975 Jul 18;18(3):205–211. doi: 10.1007/BF02889248. [DOI] [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- Moskowitz M. S. Fatty acid-induced steatosis in monolayer cell cultures. Wistar Inst Symp Monogr. 1967;6:49–62. [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule W. J., Zemplenyi T. K., Rounds D. E., Blankenhorn D. H. Light- and electron-microscopic characteristics of artrial smooth muscle cell cultures subjected to hypoxia or carbon monoxide. Atherosclerosis. 1976 Oct;25(1):111–123. doi: 10.1016/0021-9150(76)90053-8. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. L., Jr, Khairallah P. A. Arterial endothelial permeability and vascular disease. The "trap door" effect. Exp Mol Pathol. 1973 Apr;18(2):241–260. doi: 10.1016/0014-4800(73)90022-1. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D., Geyer R. P. Formation and disappearance of triglyceride droplets in strain L fibroblasts. An electron microscopic study. Exp Cell Res. 1971 Nov;69(1):193–206. doi: 10.1016/0014-4827(71)90325-9. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Infante R., Polonovski J., Caroli J. Quantitative evaluation of plasma free fatty acid uptake by the isolated perfused rat liver. Influence of hypoxia. Biochim Biophys Acta. 1971 Jul 13;239(2):154–161. doi: 10.1016/0005-2760(71)90161-5. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]