Abstract
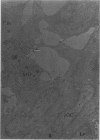
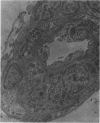
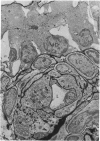
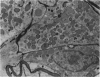
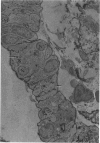
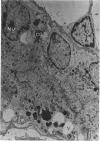
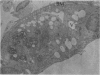
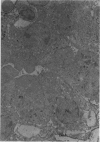
Partial ligation of the aorta between the renal arteries induces marked atrophy of the cortical tubules (including the macula densa) of the left (endocrine) kidney with a remarkable increase in the number and granularity of hypersecretory juxtaglomerular granulated cells (JGC) which are found not only at the glomerular pole of arterioles but also in the walls of arteries and arterioles far removed from the glomerulus. Staining of fine sections of Araldite-embedded endocrine kidneys according to the periodic acid-thiocarbohydrazide-silver proteinate technique of Thiery reveals abundant glycogen in the JGC and less in the blood vessels and tubules. Juxtaglomerular granules are argentaphobic, but their rim is positively stained when ultrathin sections of glutaraldehyde-fixed, glycol methacrylate-embedded kidneys are exposed to phosphotungstic acid at a low pH. A positive reaction is also shown by the cell coat and lysosomes of JGC as well as by the thickened basal lamina, cell coat, cytosomes, and cytosegresomes of the atrophic tubules. Atrophy is most pronounced in the proximal convoluted tubules, which lose their apical microvilli, their basal infoldings and the majority of their mitochondria and cytosomes.
Full text
PDF













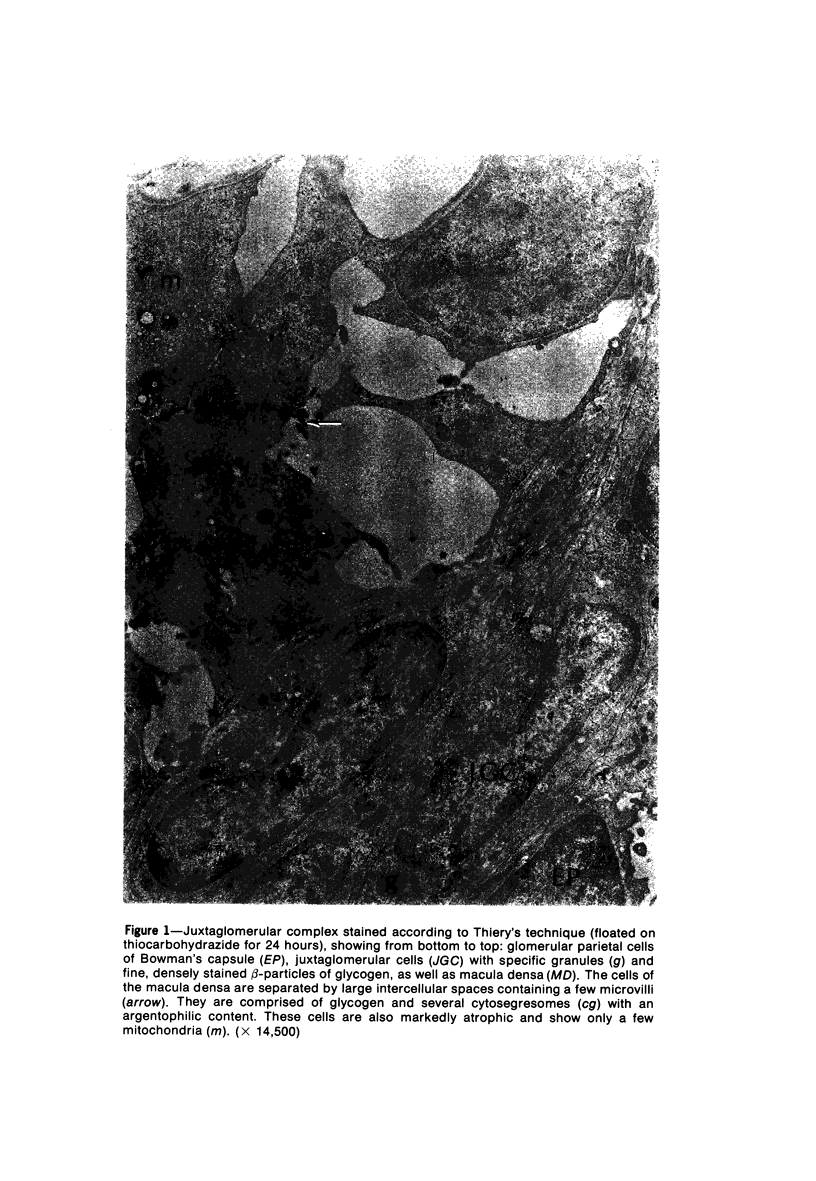
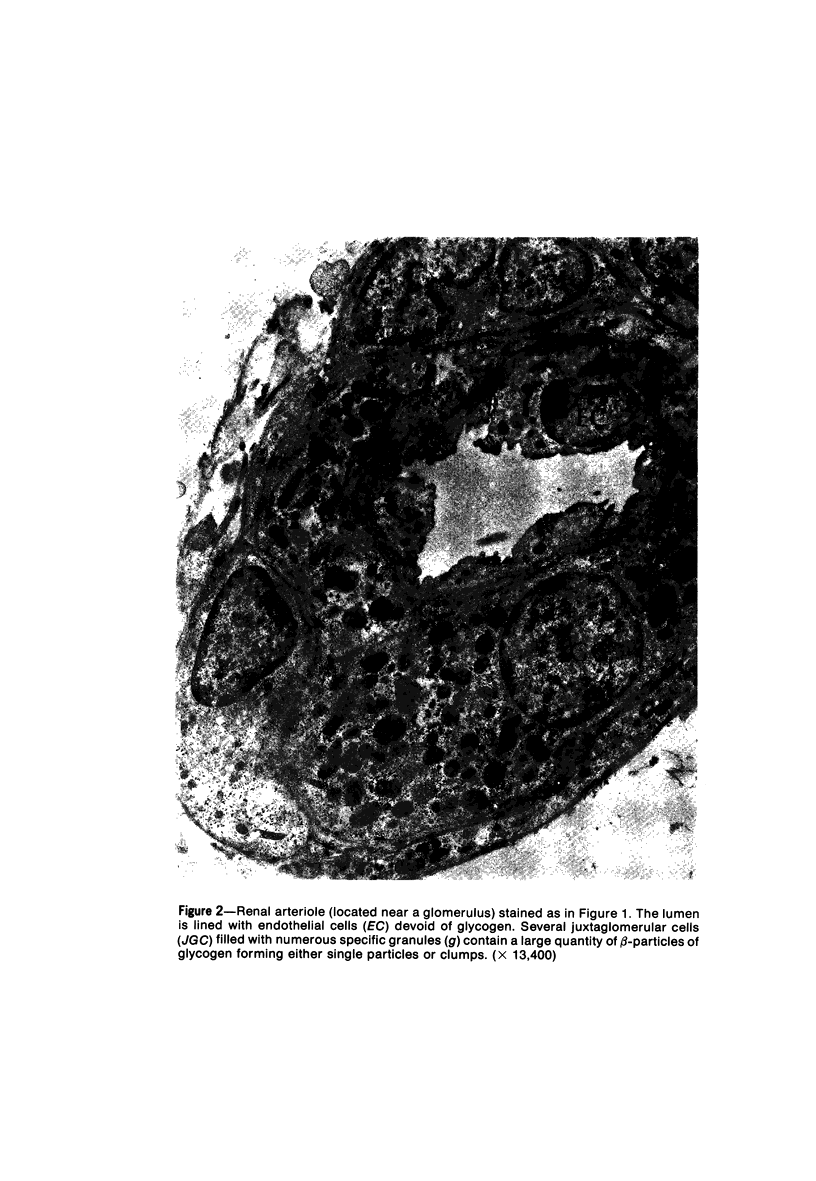
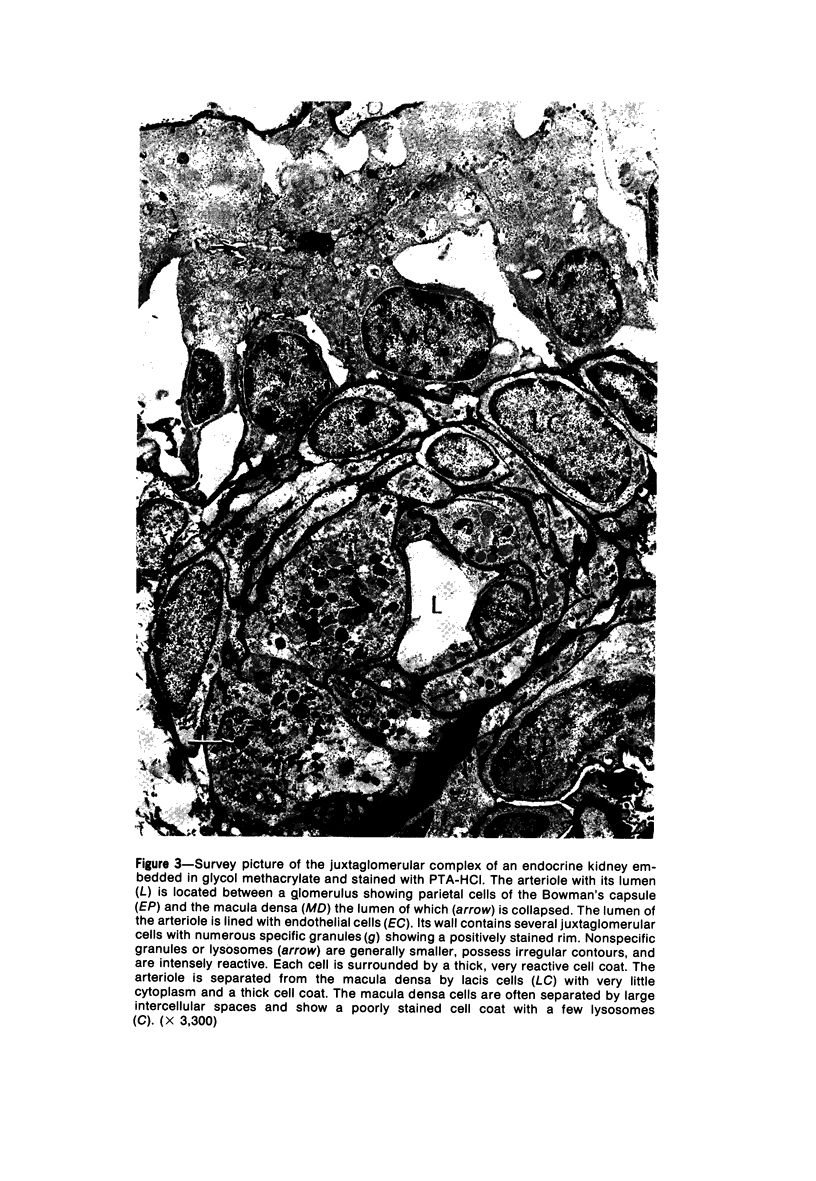
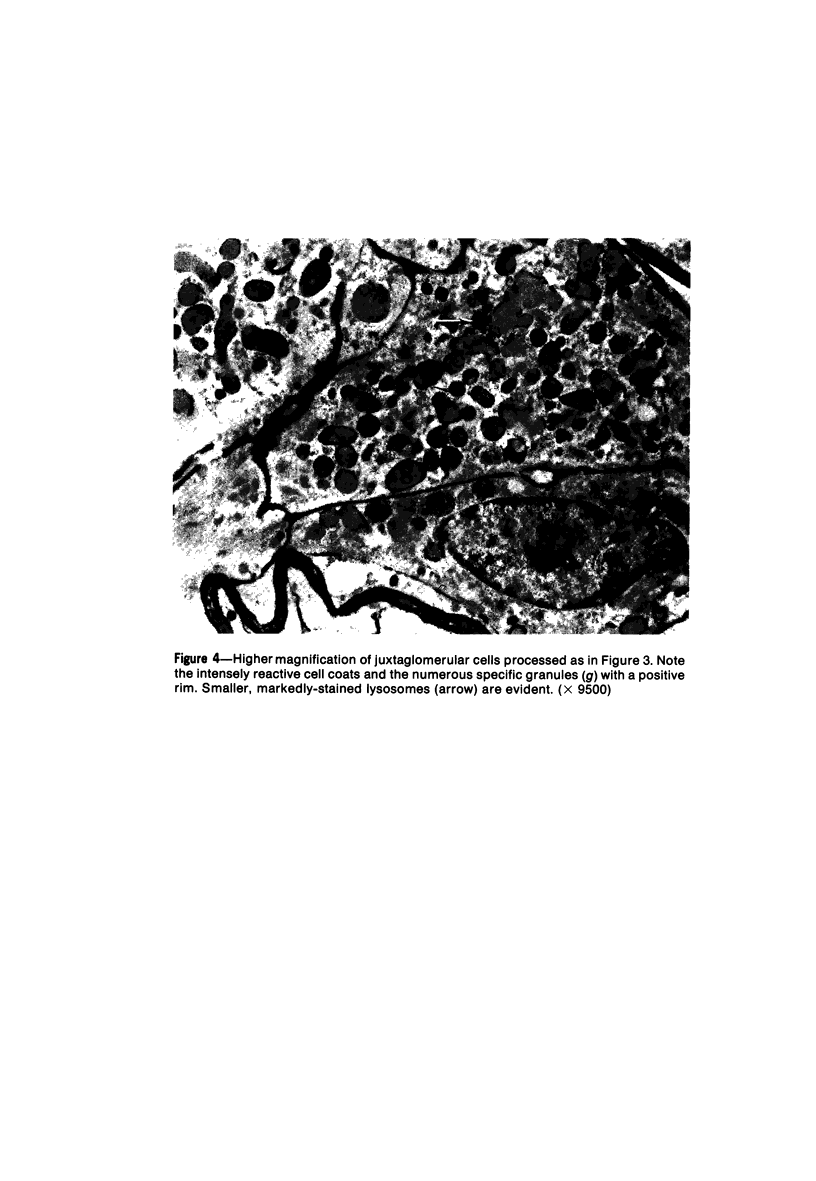
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babaï F., Bernhard W. Détection cytochimique par l'acide phosphotungstique de certains polysaccharides sur coupes á congélation ultrafines. J Ultrastruct Res. 1971 Dec;37(5):601–617. doi: 10.1016/s0022-5320(71)80028-x. [DOI] [PubMed] [Google Scholar]
- Biava C. G., West M. Fine structure of normal human juxtaglomerular cells. II. Specific and nonspecific cytoplasmic granules. Am J Pathol. 1966 Nov;49(5):955–979. [PMC free article] [PubMed] [Google Scholar]
- Biava C., Grossman A., West M. Ultrastructural observations on renal glycogen in normal and pathologic human kidneys. Lab Invest. 1966 Jan;15(1 Pt 2):330–356. [PubMed] [Google Scholar]
- Bucher O., Krstić R. Weitere ultrastrukturelle Untersuchungen an der Macula densa des Mittelstückes der Niere: Helle und dunkle Zellen. Acta Anat (Basel) 1971;78(3):335–348. [PubMed] [Google Scholar]
- Bucher O., Krstić R. Weiterer Beitrag zum Vorkommen von dunklen Zellen in der Macula densa des Mittelstückes der Ratten-Niere. Z Anat Entwicklungsgesch. 1973;141(3):319–329. [PubMed] [Google Scholar]
- CLARK S. L., Jr Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957 May 25;3(3):349–362. doi: 10.1083/jcb.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR T. B., BERTHRONG M., THOMAS W. C., Jr, HOWARD J. E. Hypertension due to unilateral renal disease; with a report on a functional test helpful in diagnosis. Bull Johns Hopkins Hosp. 1957 Jun;100(6):241–276. [PubMed] [Google Scholar]
- Cantin M., Bajusz E. Electrolyte content and ultrastructure of the kidney in hamsters with heart failure. Exp Mol Pathol. 1973 Oct;19(2):143–159. doi: 10.1016/0014-4800(73)90074-9. [DOI] [PubMed] [Google Scholar]
- Cantin M., Benchimol S., Castonguay Y., Berlinguet J. C., Huet M. Ultrastructural cytochemistry of atrial muscle cells. V. Characterization of specific granules in the human left atrium. J Ultrastruct Res. 1975 Aug;52(2):179–192. doi: 10.1016/s0022-5320(75)80110-9. [DOI] [PubMed] [Google Scholar]
- Cantin M., Benchimol S. Localization and characterization of carbohydrates in adrenal medullary cells. J Cell Biol. 1975 May;65(2):463–469. doi: 10.1083/jcb.65.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin M., Desormeaux Y., Chlebovicova J., Benchimol S., de Araujo-Nascimento M. Comparative ultrastructural cytochemistry of juxtaglomerular cell granules and renal tubular cell lysosomes. Lab Invest. 1975 Dec;33(6):648–657. [PubMed] [Google Scholar]
- Cantin M., Huet M. Histochemistry and ultrastructure of the juxtaglomerular apparatus in magnesium-deficient rats. Can J Physiol Pharmacol. 1973 Nov;51(11):835–844. doi: 10.1139/y73-128. [DOI] [PubMed] [Google Scholar]
- Cantin M., Leone A., Bajusz E. Participation of renal electrolytes in the mechanism of edema in congestive heart failure: studies on cardiomyopathic hamsters. Recent Adv Stud Cardiac Struct Metab. 1972;1:303–324. [PubMed] [Google Scholar]
- Davis J. O. What signals the kidney to release renin? Circ Res. 1971 Mar;28(3):301–306. doi: 10.1161/01.res.28.3.301. [DOI] [PubMed] [Google Scholar]
- FLUME J. B., ASHWORTH C. T., JAMES J. A. AN ELECTRON MICROSCOPIC STUDY OF TUBULAR LESIONS IN HUMAN KIDNEY BIOPSY SPECIMENS. Am J Pathol. 1963 Dec;43:1067–1087. [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., Long J. A., Jones A. L. The ultrastructure of endocrine glands. Recent Prog Horm Res. 1969;25:315–380. doi: 10.1016/b978-0-12-571125-8.50010-7. [DOI] [PubMed] [Google Scholar]
- Fisher E. R. Lysosomal nature of juxtaglomerular granules. Science. 1966 Jun 24;152(3730):1752–1753. doi: 10.1126/science.152.3730.1752. [DOI] [PubMed] [Google Scholar]
- Gomba S., Soltész B. M. Histochemistry of lysosomal enzymes in juxtaglomerular cells. Experientia. 1969 May 15;25(5):513–513. doi: 10.1007/BF01900791. [DOI] [PubMed] [Google Scholar]
- HATT P. Y., DVOJAKOVIC M., CORNET P. [Contribution of electron microscopy to the study of the mechanism of experimental arterial hypertension of renal origin. II. Renal ischemia in the rabbit]. Pathol Biol. 1962 Jan;10:23–40. [PubMed] [Google Scholar]
- Harada K. Fixative-stain sequences for selective demonstration of juxtaglomerular cells. Stain Technol. 1966 Mar;41(2):83–89. doi: 10.3109/10520296609116285. [DOI] [PubMed] [Google Scholar]
- Harada K. The demonstration of two acid groups in juxtaglomerular granules with basic triphenyl methane dyes, aldehyde-fuchsin and Schiff's reagent. Histochemie. 1967;10(1):74–87. doi: 10.1007/BF00304376. [DOI] [PubMed] [Google Scholar]
- Hess R. Renal Histochemistry of Oxidative Enzyme Systems in Aminonucleoside Nephrosis. Am J Pathol. 1960 Nov;37(5):583–597. [PMC free article] [PubMed] [Google Scholar]
- Huet M., Benchimol S., Castonguay Y., Cantin M. Ultrastructural cytochemistry of atrial muscle cells. III. Reactivity of specific granules in man. Histochemistry. 1974;41(2):87–105. doi: 10.1007/BF00499124. [DOI] [PubMed] [Google Scholar]
- Huet M., Cantin M. Ultrastructural cytochemistry of atrial muscle cells. I. Characterization of the carbohydrate content of specific granules. Lab Invest. 1974 Apr;30(4):514–524. [PubMed] [Google Scholar]
- Iglesias J. R., Bernier R., Simard R. Ultracryotomy: a routine procedure. J Ultrastruct Res. 1971 Jul;36(1):271–289. doi: 10.1016/s0022-5320(71)80104-1. [DOI] [PubMed] [Google Scholar]
- Jacobs M. M., Nyc J. F., Brown D. M. Isolation and chemical properties of a repressible acid phosphatase in Neurospora crassa. J Biol Chem. 1971 Mar 10;246(5):1419–1425. [PubMed] [Google Scholar]
- KROON D. B. Origin of the PAS-positive granulated epithelioid cells of the juxta-glomerular apparatus. Acta Anat (Basel) 1960;41:138–156. doi: 10.1159/000141612. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R., GENEST J. Study of renal ischaemic tubular atrophy in 79 patients with arterial hypertension. Can Med Assoc J. 1960 Jun 18;82:1249–1253. [PMC free article] [PubMed] [Google Scholar]
- MASSON G. M., YAGI S., KASHII C., FISHER E. R. FURTHER OBSERVATIONS ON JUXTAGLOMERULAR CELLS AND RENAL PRESSOR ACTIVITY IN EXPERIMENTAL HYPERTENSION. Lab Invest. 1964 Apr;13:321–330. [PubMed] [Google Scholar]
- Mandalenakis N., Cantin M., Dumont A. L'appareil juxtaglomérulaire du rein endocrinien. Etude histologique et ultrastructurale. Pathol Biol (Paris) 1970 Mar;18(5):233–240. [PubMed] [Google Scholar]
- Nawar T., Lefebvre R., Rojo-Ortega J. M., Cartier P., Genest J. Reversal of ischemic tubular atrophy. Ann Intern Med. 1970 Apr;72(4):529–532. doi: 10.7326/0003-4819-72-4-529. [DOI] [PubMed] [Google Scholar]
- OLIVER J., MacDOWELL M., TRACY A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest. 1951 Dec;30(121):1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMOS J., SOLBACH H. G. [Contribution to the morphology of the kidney in diabetes mellitus]. Frankf Z Pathol. 1963;72:379–408. [PubMed] [Google Scholar]
- Pease D. C. Phosphotungstic acid as a specific electron stain for complex carbohydrates. J Histochem Cytochem. 1970 Jun;18(6):455–458. doi: 10.1177/18.6.455-a. [DOI] [PubMed] [Google Scholar]
- Pease D. C. Polysaccharides associated with the exterior surface of epithelial cells: kidney, intestine, brain. J Ultrastruct Res. 1966 Aug;15(5):555–588. doi: 10.1016/s0022-5320(66)80128-4. [DOI] [PubMed] [Google Scholar]
- Plapp B. V., Cole R. D. Purification and characterization of bovine liver beta-glucuronidase. Arch Biochem Biophys. 1966 Sep 26;116(1):193–206. doi: 10.1016/0003-9861(66)90027-0. [DOI] [PubMed] [Google Scholar]
- Quintarelli G., Bellocci M., Geremia R. On phosphotungstic acid staining. IV. Selectivity of the staining reaction. J Histochem Cytochem. 1973 Feb;21(2):155–160. doi: 10.1177/21.2.155. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Hernandez W., Leblond C. P. Detection of complex carbohydrates in the Golgi apparatus of rat cells. J Cell Biol. 1969 Feb;40(2):395–414. doi: 10.1083/jcb.40.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K. A., Ganote C. E., Jennings R. B. Alterations in renal cortex following ischemic injury. 3. Ultrastructure of proximal tubules after ischemia or autolysis. Lab Invest. 1972 Apr;26(4):347–363. [PubMed] [Google Scholar]
- Rojo-Ortega J. M., Rosenthal J., Boucher R., Genest J. Effects of high temperature and acute diuresis on glucose-6-phosphate dehydrogenase activity of mascula densa and plasma renin activity in rats. Can J Physiol Pharmacol. 1969 Oct;47(10):837–840. doi: 10.1139/y69-139. [DOI] [PubMed] [Google Scholar]
- SULSER F., GROSS F. Pressorische Substanzen in den Nieren von Ratten mit sogenannter Endocrine Kidney nach Selye. Helv Physiol Pharmacol Acta. 1956;14(2):C45–C47. [PubMed] [Google Scholar]
- Salzgeber B., Weber R. La régression du mésonéphros chez l'embryon de poulet. Etude des activités de la phosphatase acide et des cathepsines. Analyse biochimique, histochimique et observations au microscope électronique. J Embryol Exp Morphol. 1966 Jun;15(3):397–419. [PubMed] [Google Scholar]
- Scott J. E., Glick D. The invalidity of "phosphotungstic acid as a specific electron stain for complex carbohydrates". J Histochem Cytochem. 1971 Jan;19(1):63–64. doi: 10.1177/19.1.63. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Phosphotungstate: a "universal" (nonspecific) precipitant for polar polymers in acid solution. J Histochem Cytochem. 1971 Nov;19(11):689–691. doi: 10.1177/19.11.689. [DOI] [PubMed] [Google Scholar]
- VANDER A. J., MILLER R. CONTROL OF RENIN SECRETION IN THE ANESTHETIZED DOG. Am J Physiol. 1964 Sep;207:537–546. doi: 10.1152/ajplegacy.1964.207.3.537. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA F., SEKIGUCHI T. CYTOLOGICAL STUDIES ON THE POSSIBLE SITES OF RENIN FORMATION IN HYPERTENSIVE RATS BY DOCA, CORTISONE AND THYROXINE. Endocrinol Jpn. 1963 Jun;10:102–118. doi: 10.1507/endocrj1954.10.102. [DOI] [PubMed] [Google Scholar]