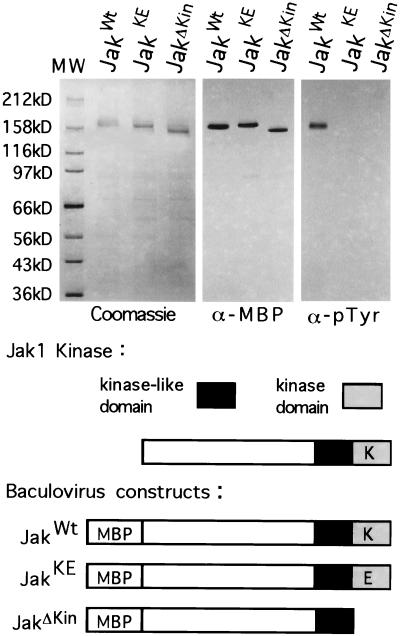
Figure 3.
Analysis of the kinase activity of wild-type and mutant Jak1 kinases. Wild-type and mutant Jak1 kinase genes were cloned into a baculovirus expression vector and expressed as a fusion protein with maltose binding protein (MBP), to aid in purification. The JakKE mutant possesses a point mutation at position 2915, changing a lysine (K) to a glutamic acid (E). The JakΔKin mutant was constructed by deleting the C-terminal kinase domain. The fusion proteins were purified to near homogeneity on amylose resin from infected Sf9 cells, and showed little degradation as revealed by Coomassie blue staining and Western analysis with anti-MBP antisera. Purified fusion proteins were analyzed for the presence of phosphotyrosine resulting from autophosphorylation. Only the wild-type kinase possesses phosphotyrosine.