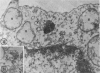
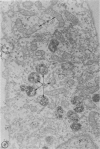
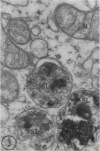
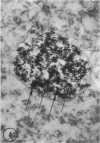
Abstract
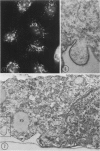
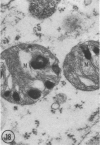
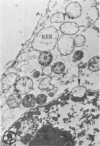
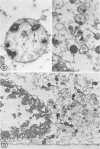
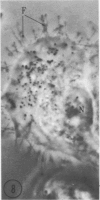
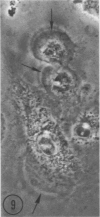
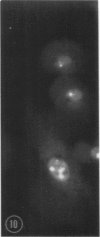
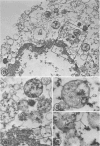
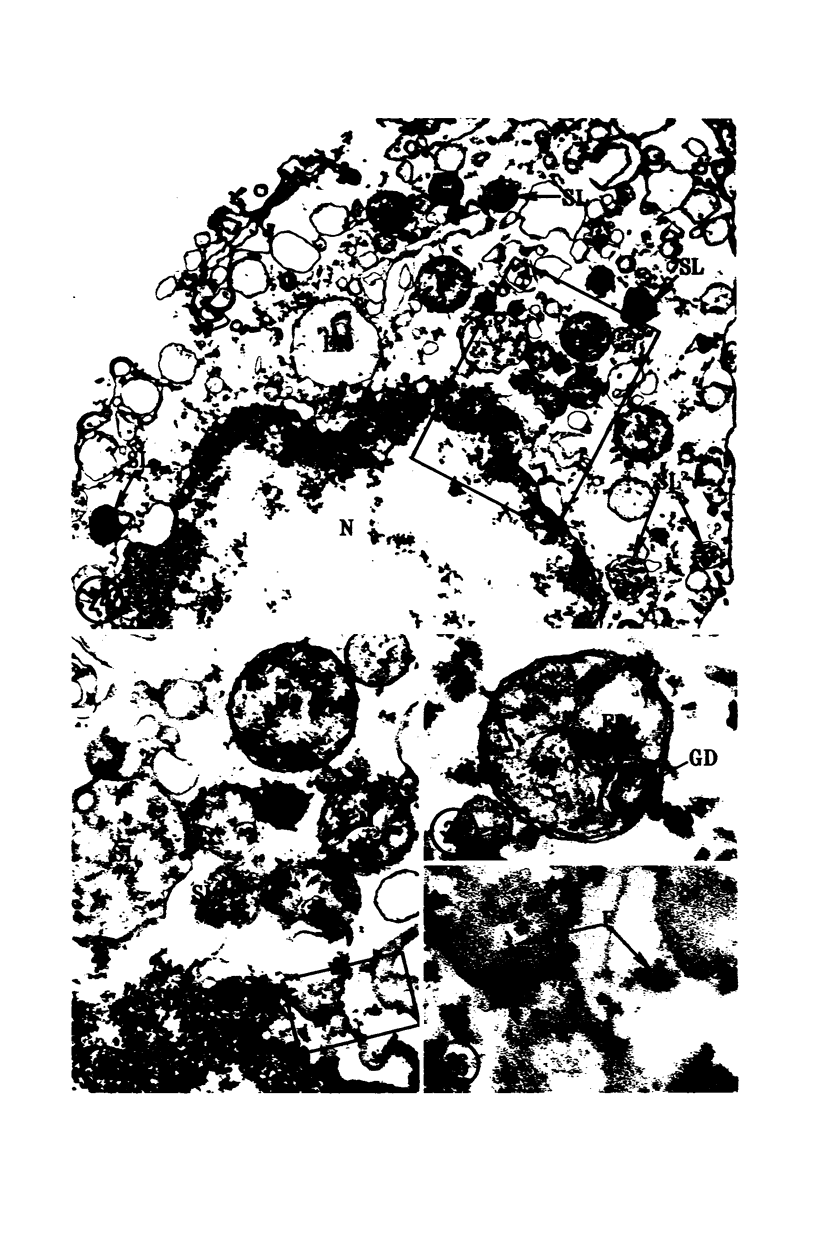
In two types of cell injury in a tissue culture system, the possibility was tested that lysosome rupture may be a lethal cellular reaction to injury, and thus an important general cause of irreversibility of damage in injured tissue. Prior labeling of secondary lysosomes with the fluorochrome acridine orange, or with ferritin, was used to trace changes in lysosomes after applying an injury. The metabolic inhibitors iodoacetate and cyanide were used together to block the cell's energy supply, or attachment of antiserum and subsequent complement attack were used to damage the surface membrane, producing rapid loss of cell volume control. Living cells were studied by time-lapse phase-contrast cinemicrography and fluorescence microscopy, and samples were fixed at intervals for electron microscopy. The cytolytic action of complement was lethal to sensitized cells within 2 hours, but results showed that lysosomes did not rupture for approximately 4 hours and in fact did not release the fluorescent dye until after reaching the postmortem necrotic phase of injury. Cells treated with metabolic inhibitors also showed irreversible alterations, while lysosomes remained intact and retained the ferritin marker. The fluorochrome marker, acridine orange, escaped from lysosomes early after metabolic injury, but the significance of this observation is not clear. The results are interpreted as evidence against the concept that lysosome rupture threatens the survival of injured cells. The original suicide bag mechanism of cell damage thus is apparently not operative in the systems studied. Lysosomes appear to be relatively stable organelles which, following injury of the types studied, burst only after cell death, acting then as scavengers which help to clear cellular debris.
Full text
PDF























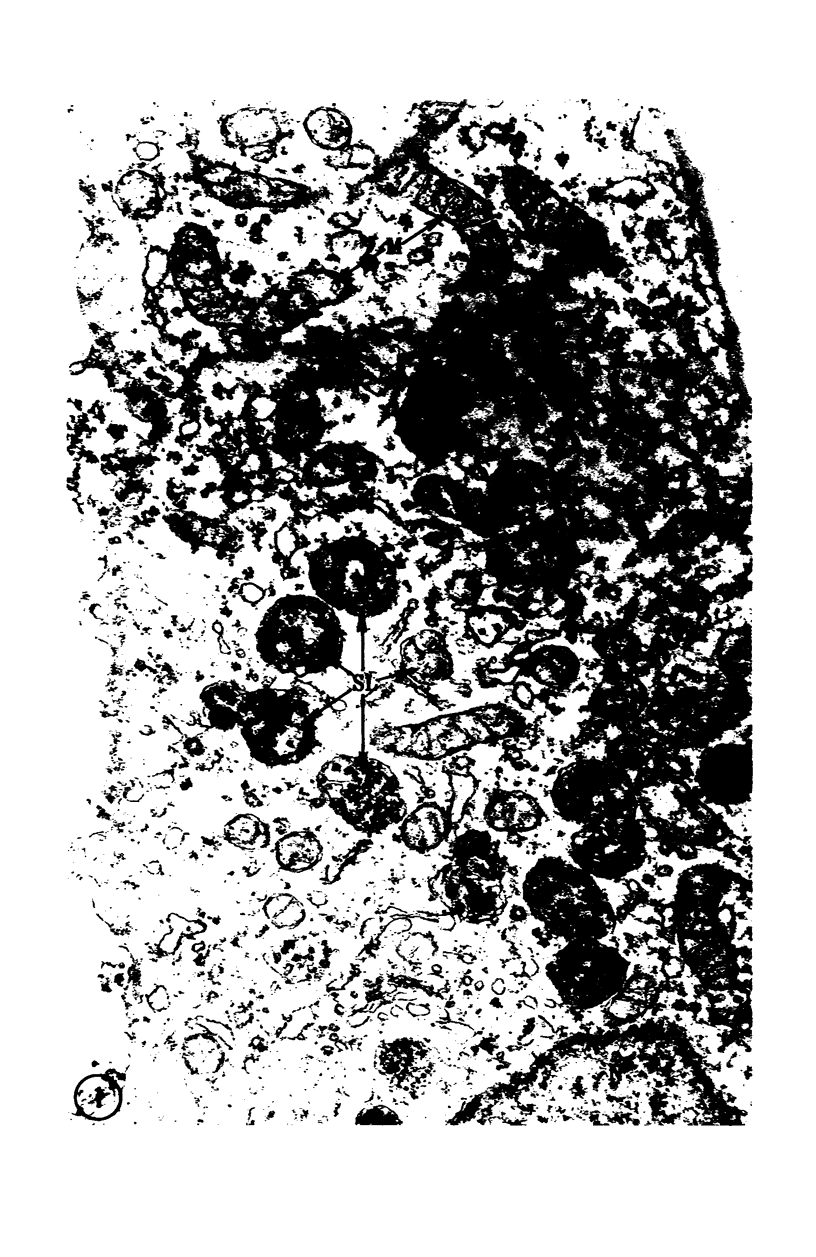
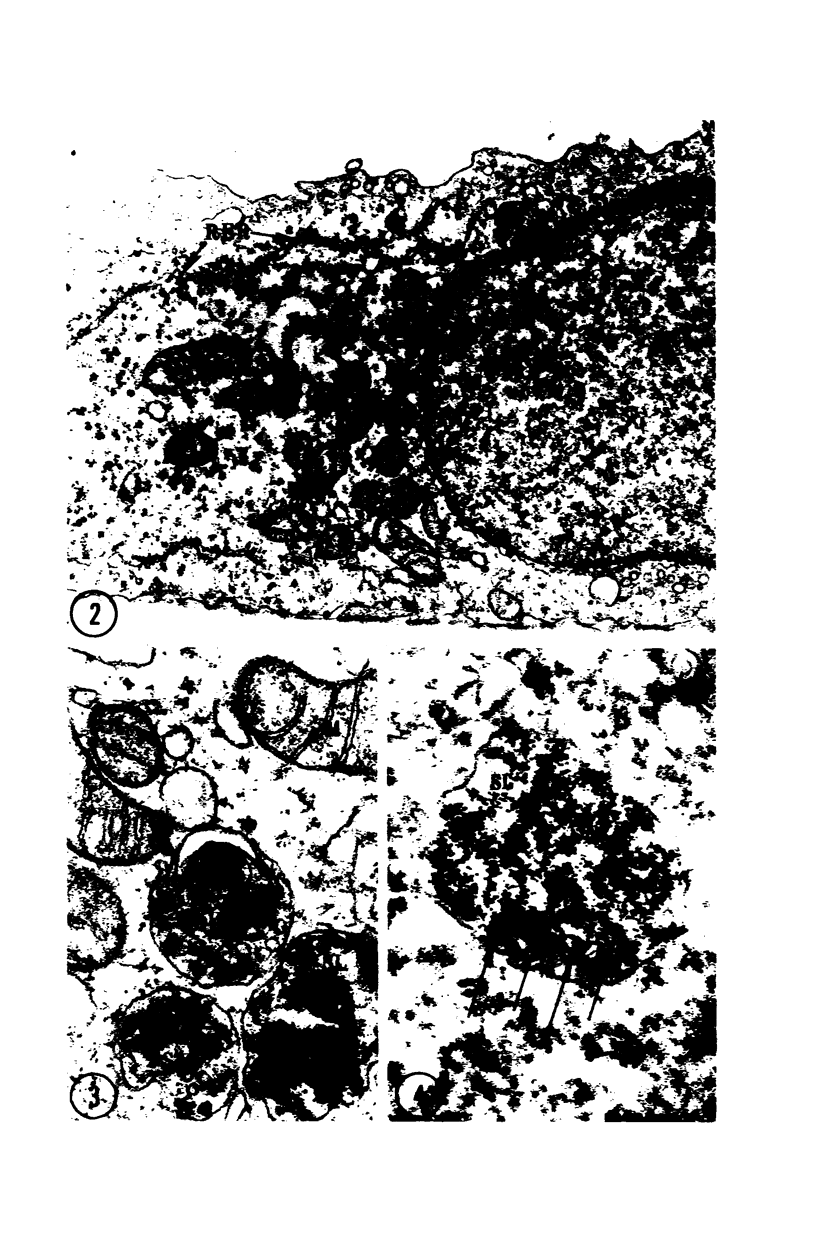
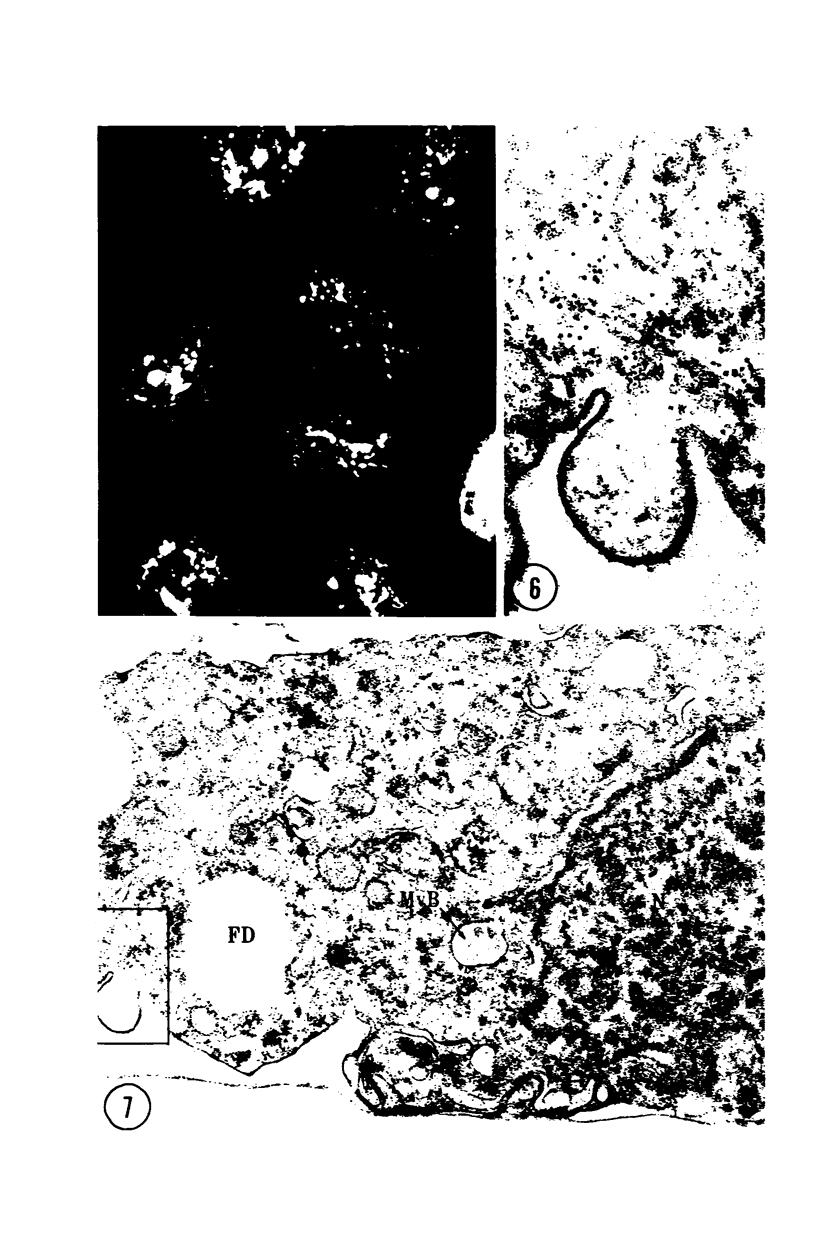
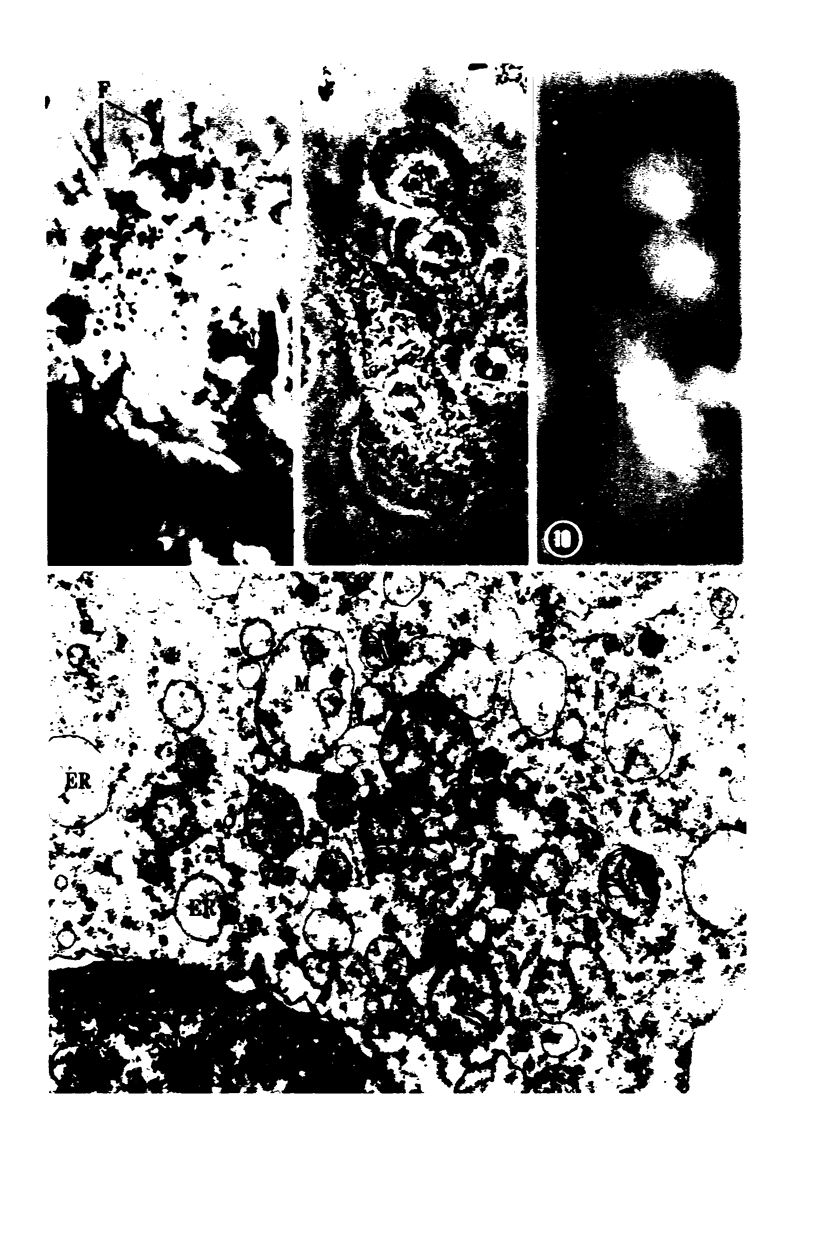
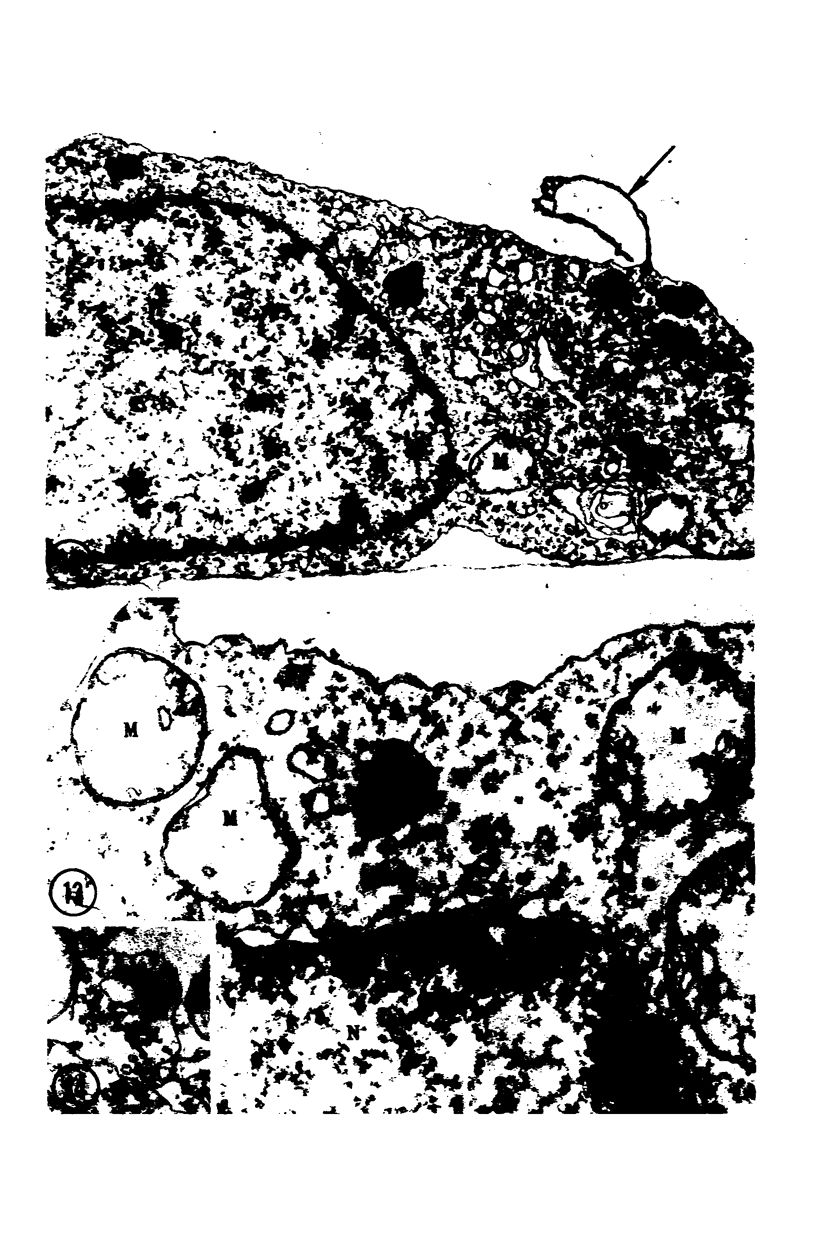
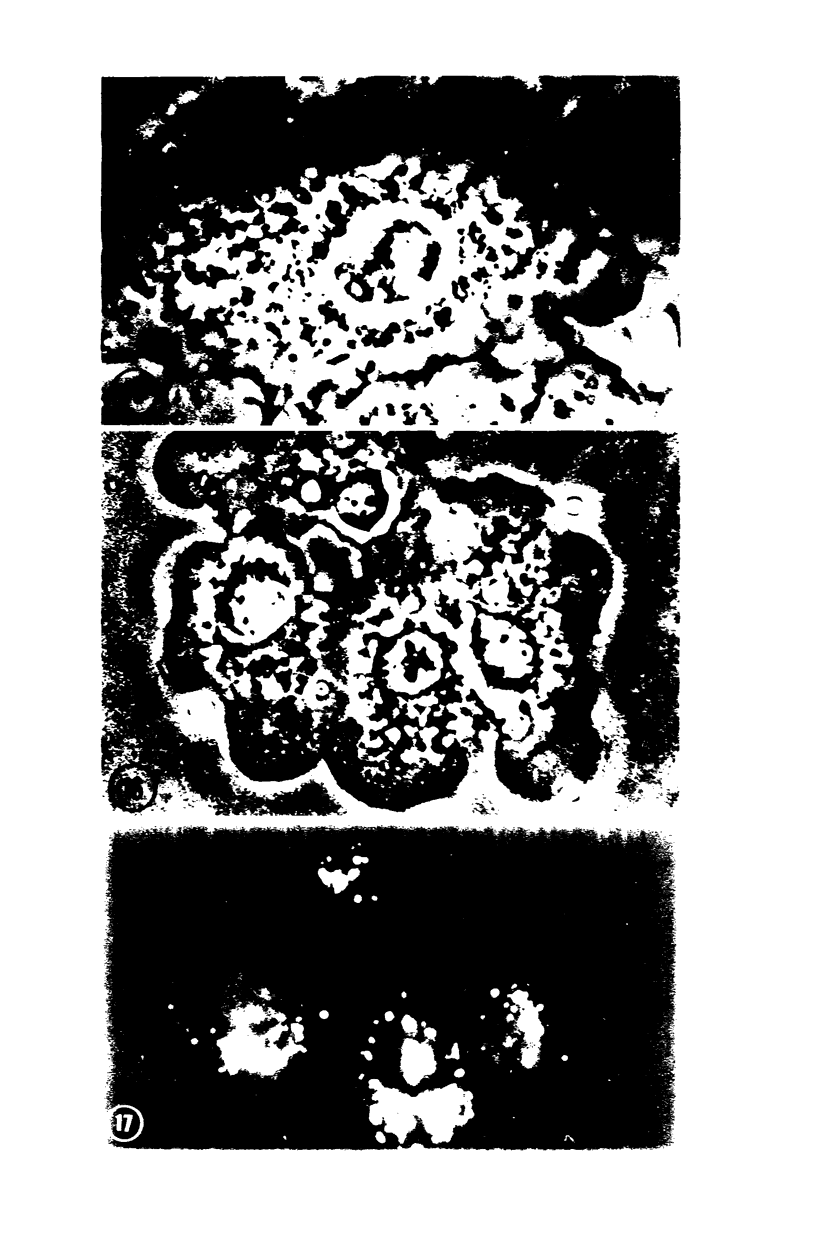
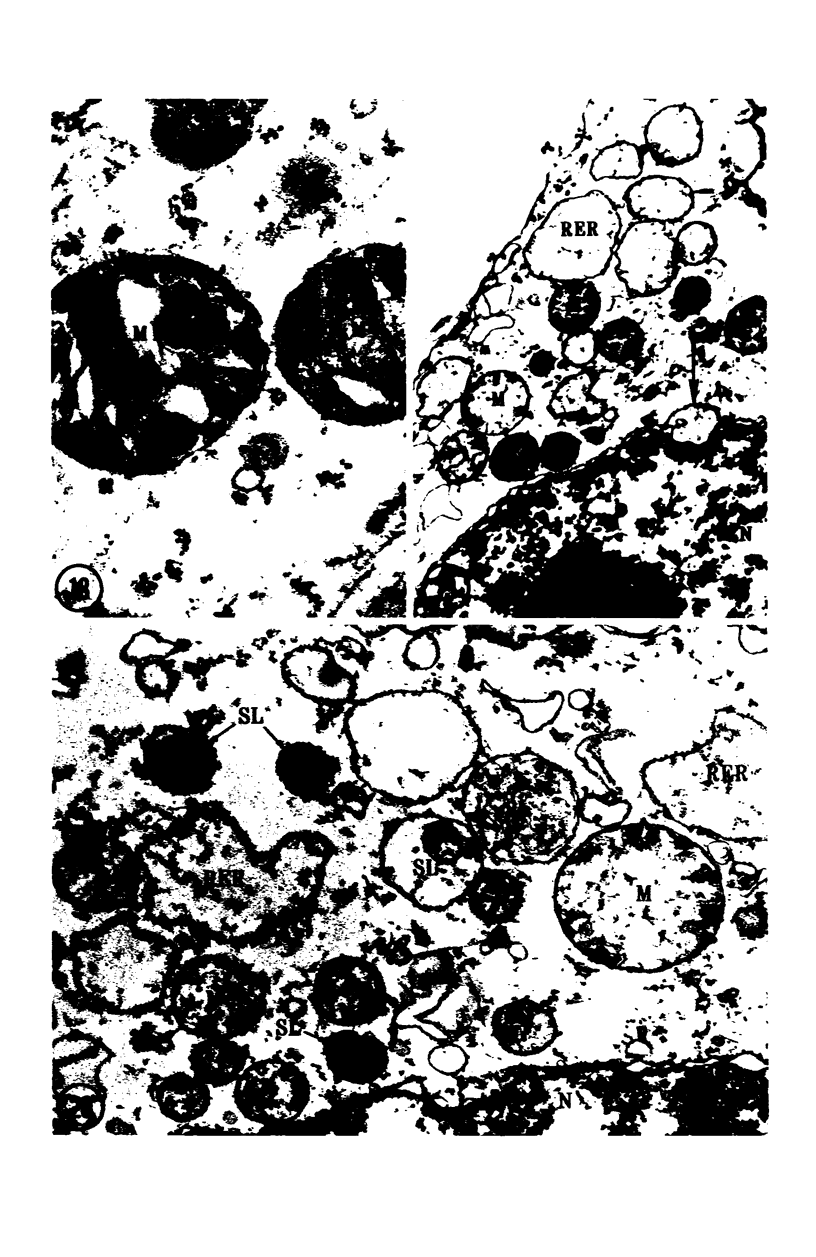

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. HISTOCHEMICAL STUDIES OF LYSOSOMES AND LYSOSOMAL ENZYMES IN VIRUS-INFECTED CELL CULTURES. J Exp Med. 1965 Mar 1;121:463–476. doi: 10.1084/jem.121.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., YOUNG M. R. UPTAKE OF DYES AND DRUGS BY LIVING CELLS IN CULTURE. Life Sci. 1964 Dec;3:1407–1414. doi: 10.1016/0024-3205(64)90082-7. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Magnus I. A., Young M. R. Role of lysosomes and of cell membranes in photosensitization. Nature. 1966 Feb 26;209(5026):874–878. doi: 10.1038/209874a0. [DOI] [PubMed] [Google Scholar]
- Arstila A. U., Jauregui H. O., Chang J., Trump B. F. Studies on cellular autophagocytosis. Relationship between heterophagy and autophagy in HeLa cells. Lab Invest. 1971 Feb;24(2):162–174. [PubMed] [Google Scholar]
- BEAUFAY H., DE DUVE C. Tissue fractionation studies. 9. Enzymic release of bound hydrolases. Biochem J. 1959 Dec;73:604–609. doi: 10.1042/bj0730604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barankay T., Horpácsy G., Nagy S., Petri G. Changes in the level of lysosomal enzymes in plasma and lymph in hemorrhagic shock. Med Exp Int J Exp Med. 1969;19(5):267–271. doi: 10.1159/000137208. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., De Duve C. Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J Cell Biol. 1965 Jul;26(1):219–243. doi: 10.1083/jcb.26.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers W. E., de Duve C. Lysosomes in lymphoid tissue. II. Intracellular distribution of acid hydrolases. J Cell Biol. 1967 Feb;32(2):339–348. doi: 10.1083/jcb.32.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R. S. M. Continuous subcultivation of epithelial-like cells from normal human tissues. Proc Soc Exp Biol Med. 1954 Nov;87(2):440–443. doi: 10.3181/00379727-87-21406. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The influence of phagocytosis on the intracellular distribution of granule-associated components of polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:1015–1022. doi: 10.1084/jem.112.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico P. G., Bird J. W. The use of acridine orange as a lysosomal marker in rat skeletal muscle. J Cell Biol. 1969 Nov;43(2):367–371. doi: 10.1083/jcb.43.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter R. L., De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967 May;33(2):437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERICSSON J. L., TRUMP B. F. ELECTRON MICROSCOPIC STUDIES OF THE EPITHELIUM OF THE PROXIMAL TUBULE OF THE RAT KIDNEY. I. THE INTRACELLULAR LOCALIZATION OF ACID PHOSPHATASE. Lab Invest. 1964 Nov;13:1427–1456. [PubMed] [Google Scholar]
- Ericsson J. L., Biberfeld P., Seljelid R. Electron microscopic and cytochemical studies of acid phosphatase and aryl sulfatase during autolysis. Acta Pathol Microbiol Scand. 1967;70(2):215–228. doi: 10.1111/j.1699-0463.1967.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Ericsson J. L., Biberfeld P. Studies on aldehyde fixation. Fixation rates and their relation to fine structure and some histochemical reactions in liver. Lab Invest. 1967 Sep;17(3):281–298. [PubMed] [Google Scholar]
- GOLDBLATT P. J., TRUMP B. F., STOWELL R. E. STUDIES ON NECROSIS OF MOUSE LIVER IN VITRO: ALTERATIONS IN SOME HISTOCHEMICALLY DEMONSTRABLE HEPATOCELLULAR ENZYMES. Am J Pathol. 1965 Aug;47:183–208. [PMC free article] [PubMed] [Google Scholar]
- GREEN H., FLEISCHER R. A., BARROW P., GOLDBERG B. The cytotoxic action of immune gamma globulin and complement on Krebs ascites tumor cells. II. Chemical studies. J Exp Med. 1959 May 1;109(5):511–521. doi: 10.1084/jem.109.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn F. L., Shelburne J. D., Trump B. F. Disorders of cell volume regulation. I. Effects of inhibition of plasma membrane adenosine triphosphatase with ouabain. Am J Pathol. 1968 Dec;53(6):1041–1071. [PMC free article] [PubMed] [Google Scholar]
- Griffin C. C., Waravdekar V. S., Trump B. F., Goldblatt P. J., Stowell R. E. Studies on necrosis of mouse liver in vitro. Alterations in activities of succinoxidase, succinic dehydrogenase, glutamic dehydrogenase, acid phosphatase, uricase, glucose-6-phosphatase and NAD-pyrophosphorylase. Am J Pathol. 1965 Nov;47(5):833–850. [PMC free article] [PubMed] [Google Scholar]
- Gritzka T. L., Trump B. F. Renal tubular lesions caused by mercuric chloride. Electron microscopic observations: degeneration of the pars recta. Am J Pathol. 1968 Jun;52(6):1225–1277. [PMC free article] [PubMed] [Google Scholar]
- HERDSON P. B., SOMMERS H. M., JENNINGS R. B. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF NORMAL AND ISCHEMIC DOG MYOCARDIUM WITH SPECIAL REFERENCE TO EARLY CHANGES FOLLOWING TEMPORARY OCCLUSION OF A CORONARY ARTERY. Am J Pathol. 1965 Mar;46:367–386. [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homewood C. A., Warhurst D. C., Peters W., Baggaley V. C. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972 Jan 7;235(5332):50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Herdson P. B., Sommers H. M. Structural and functional abnormalities in mitochondria isolated from ischemic dog myocardium. Lab Invest. 1969 Jun;20(6):548–557. [PubMed] [Google Scholar]
- Jonsson J., Fagraeus A., Biberfeld G. The mixed haemadsorption test as an aid to the diagnosis of thyroid autoimmune disease. Clin Exp Immunol. 1968 May;3(4):287–304. [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., LA GATTUTA M., THOMPSON T. E. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med. 1960;333:421–465. doi: 10.1007/BF00955327. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I. DYNAMICS OF ACRIDINE ORANGE-CELL INTERACTION. I. INTERRELATIONSHIPS OF ACRIDINE ORANGE PARTICLES AND CYTOPLASMIC REDDENING. J Cell Biol. 1963 Aug;18:237–250. doi: 10.1083/jcb.18.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I., GONATAS N. K. DYNAMICS OF ACRIDINE ORANGE-CELL INTERACTION. II. DYE-INDUCED ULTRASTRUCTURAL CHANGES IN MULTIVESICULAR BODIES (ACRIDINE ORANGE PARTICLES). J Cell Biol. 1964 Apr;21:49–62. doi: 10.1083/jcb.21.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. B. The synthesis and hydrolysis of sulfate esters. Adv Enzymol Relat Subj Biochem. 1960;22:205–235. doi: 10.1002/9780470122679.ch5. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S. Liver parenchymal cell injury. 3. The nature of calcium--associated electron-opaque masses in rat liver mitochondria following poisoning with carbon tetrachloride. J Cell Biol. 1965 Jun;25(3 Suppl):53–75. doi: 10.1083/jcb.25.3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigler R., Jr Microfluorometric characterization of intracellular nucleic acids and nucleoproteins by acridine orange. Acta Physiol Scand Suppl. 1966;267:1–122. [PubMed] [Google Scholar]
- SYKES J. A., MOORE E. B. A new chamber for tissue culture. Proc Soc Exp Biol Med. 1959 Jan;100(1):125–127. doi: 10.3181/00379727-100-24546. [DOI] [PubMed] [Google Scholar]
- Schumacher H. R., Phelps P. Sequential changes in human polymorphonuclear leukocytes after urate crystal phagocytosis. An electron microscopic study. Arthritis Rheum. 1971 Jul-Aug;14(4):513–526. doi: 10.1002/art.1780140411. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Bulger R. E. New ultrastructural characteristics of cells fixed in a glutaraldehyde-osmium tetroxide mixture. Lab Invest. 1966 Jan;15(1 Pt 2):368–379. [PubMed] [Google Scholar]
- Trump B. F., Bulger R. E. Studies of cellular injury in isolated flounder tubules. IV. Electron microscopic observations of changes during the phase of altered homeostasis in tubules treated with cyanide. Lab Invest. 1968 Jun;18(6):731–739. [PubMed] [Google Scholar]
- Trump B. F., Goldblatt P. J., Stowell R. E. Studies of necrosis in vitro of mouse hepatic parenchymal cells. Ultrastructural and cytochemical alterations of cytosomes, cytosegresomes, multivesicular bodies, and microbodies and their relation to the lysosome concept. Lab Invest. 1965 Nov;14(11):1946–1968. [PubMed] [Google Scholar]
- Vasington F. D., Greenawalt J. W. Osmotically lysed rat liver mitochondria. Biochemical and ultrastructural properties in relation to massive ion accumulation. J Cell Biol. 1968 Dec;39(3):661–675. doi: 10.1083/jcb.39.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUCKER-FRANKLIN D., HIRSCH J. G. ELECTRON MICROSCOPE STUDIES ON THE DEGRANULATION OF RABBIT PERITONEAL LEUKOCYTES DURING PHAGOCYTOSIS. J Exp Med. 1964 Oct 1;120:569–576. doi: 10.1084/jem.120.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]