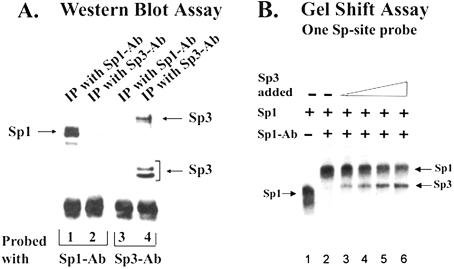
Figure 5.
Sp3 does not form heterodimers with Sp1. (A) The Sp3 antibody does not co-immunoprecipitate Sp1 or vice versa. Aliquots of the heparin agarose fraction containing most of the Sp-like activity (200 µg) were separately immunoprecipitated with Sp1 antibody or Sp3 antibody. The immunoprecipitates were eluted with SDS sample buffer and subjected to western blot analysis. The Sp1 and Sp3 immunoprecipitates were probed with Sp1 antibody (Left panel, lanes 1–2) or Sp3 antibody (right panel, lanes 3–4). (B) Gel Shift assay. A low concentration of purified Sp1 (1.2 ng) was incubated with the 32P-labeled BCAT-1 probe to generate a predominantly monomeric complex of Sp1 (lane1). Sp1 antibody (1 µl) was added to the reaction mixture to separate the Sp1 and Sp3 complexes (Lanes 2–6). In lanes 3–6, increasing levels of purified Sp3 protein (1.2, 2.4, 6.0 and 12 ng) were added, and Sp3 formed a complex with the probe that migrates differently from the Sp1/Sp1-Ab complex. About a 50% reduction in the level of Sp1/Sp1-Ab complex was observed following the addition of 12 ng of Sp3 (lane 6).