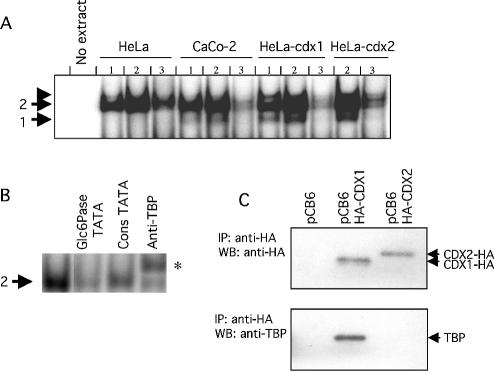
Figure 4.
Specific binding of CDX1 to the TATA box of the Glc6Pase promoter and interaction with the TATA-binding protein. (A) Gel shift assays were carried out with 5 (lanes 1) or 15 µg (lanes 2 and 3) of whole cell extracts from HeLa cells, differentiated Caco-2 cells, CDX1-expressing HeLa cells, or CDX2-expressing HeLa cells and double-strand radiolabelled oligonucleotide matching the Glc6Pase TATAAAA sequence. Control was performed without protein (no extract). Competition experiments were performed in the presence of 100-fold excess of unlabelled Glc6Pase TATAAAA oligonucleotide (lanes 3). ‘1’ and ‘2’ on the left refer to DNA–protein complexes. The arrowhead above 2 indicates the presence of a non-reproducible complex. (B) Gel shift assays were carried out with 15 µg of whole cell extract from HeLa cells and double-strand radiolabelled oligonucleotide matching the Glc6Pase TATAAAA sequence. Competition experiments were performed with 100-fold excess of unlabelled oligonucleotides (Glc6Pase TATAAAA and consTATA box). Supershift experiment was performed with anti-TBP antibody, and indicated by an asterisk. (C) CDX1 and TBP interaction. HTC116 cells were co-transfected with vehicle alone (pCB6 vector), HA-CDX1 expressing vector (pCB6-HA-CDX1) or HA-CDX2 expressing vector (pCB6-HA-CDX2) and TBP expressing vector (pXJ41-hTBP plasmid). Immunoprecipitation (IP) was performed with anti-HA antibody and revealed by western blotting (WB) with anti-HA (upper panel) or anti-TBP (lower panel).