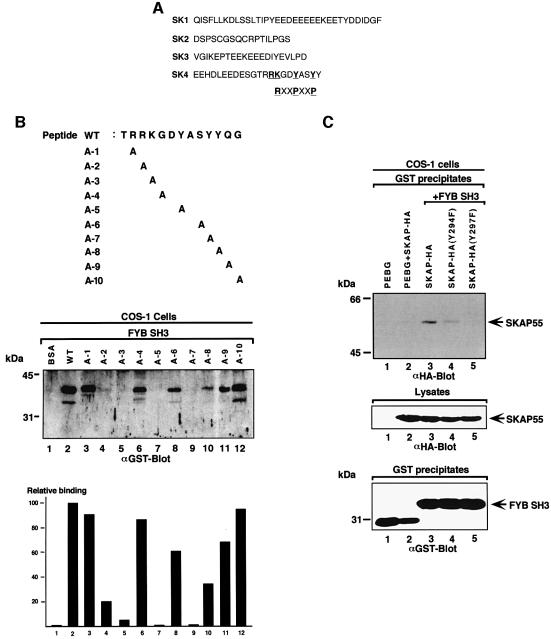
Fig. 2. Key residues are required for RKGDTASYY motif binding to the FYB SH3 domain. (A) Amino acid sequence of peptides corresponding to SK1–SK4. The SK4 region, carrying the RKGDYASY motif, is compared with a classic class I RxxPxxP. (B, upper panel) The SKAP55 peptide (TRRKGDYASYYQG; residues 288–300) used in precipitation studies. Various sequential mutants were synthesized in which alanine was used to substitute for amino acids. (Middle panel) AminoLink-Plus coupled to various peptides was used to precipitate the GST-tagged FYB SH3 domain from lysates from COS-1 cells. GST–FYB SH3 domain was detected by anti-GST immunoblotting. Lane 1, bovine serum albumin; lane 2, wild-type TRAKGDYASYYQG peptide; lane 3, TARKGDYASYYQG A-1 peptide; lane 4, TRAKGDYASYYQG A-2 peptide; lane 5, TRRAGDYASYYQG A-3 peptide;; lane 6, TRRKADYASYYQG A-4 peptide; lane 7, TRRKGDAASYYQG A-5 peptide; lane 8, TRRKGDYAAYYQG A-6 peptide; lane 9, TRRKGDYASAYQG A-7 peptide; lane 10, TRRKGDYASYAQG A-8 peptide; lane 11, TRRKGDYASYYAG A-9 peptide; lane 12, TRRKGDYASYYQA A-10 peptide. (Lower panel) Densitometric profile of SKAP55 in GST–FYB SH3 precipitates using a Scantjet laser scanner (Hewlett-Packard). (C) (Upper panel) Mutation of tyrosine residues Y294F/Y297F attenuates in vivo SKAP55 binding to the FYB SH3 domain. COS-1 cells were transfected with SKAP55, SKAP55(Y294F), SKAP55(Y297F) and FYB SH3 domain and assessed for complex formation. Glutathione–Sepharose beads were used to precipitate the GST-tagged FYB SH3 domain. Co-precipitated HA-tagged SKAP55 was detected by anti-HA immunoblotting. Lane 1, pEBG; lane 2, pEBG plus SKAP55; lane 3, SKAP55 plus FYB SH3; lane 4, SKAP55 (Y294F) plus FYB SH3; lane 5, SKAP55 (Y294F) plus FYB SH3 domain. The precipitates were separated on a 10% SDS–polyacrylamide gel and subjected to anti-HA blotting. (Middle panel) Levels of SKAP-HA protein expression. Cell lysates were separated by SDS–PAGE, transferred to nitrocellulose and subjected to blotting with anti-HA. (Lower panel) Levels of GST fusion protein expression. As in middle panel, except that lysates were blotted with anti-GST.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.