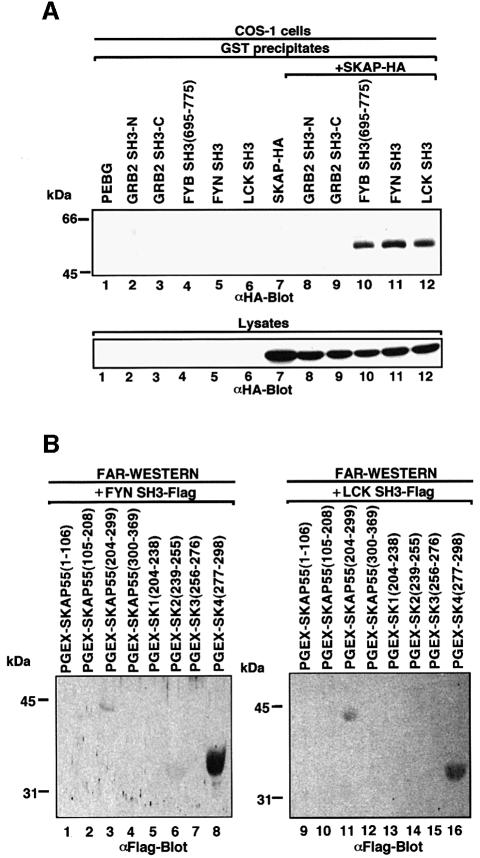
Fig. 3. Other SH3 domains capable of recognizing proline-based class I motifs also recognize tyrosine-based motifs. (A; upper panel) Co-expressed FYN and LCK SH3 domains also interact with HA-tagged SKAP55. COS-1 cells were transfected with SKAP55, GRB-2 SH3, FYB SH3, FYN SH3 and LCK SH3 in pEBG and assessed for complex formation. Glutathione beads were used to precipitate the GST fusion proteins, while co-precipitated HA-tagged SKAP55 was detected by anti-HA blotting. Lane 1, pEBG; lane 2, GRB-2 N-terminal SH3; lane 3, GRB-2 C-terminal SH3; lane 4, FYB SH3; lane 5, FYN SH3; lane 6, LCK SH3; lane 7, SKAP55-HA; lane 8, GRB-2 N-terminal SH3 and SKAP55-HA; lane 9, GRB-2 C-terminal SH3 and SKAP55-HA; lane 10, FYB SH3 and SKAP55-HA; lane 11, FYN SH3 and SKAP55-HA; lane 12, LCK SH3 and SKAP55-HA. The precipitates were separated on a 10% SDS–polyacrylamide gel, transferred to nitrocellulose and subjected to anti-HA blotting. (Lower panel) Expression of SKAP55. As in upper panel except that cell lysate was run on gel and subjected to anti-HA blotting. (B) In vitro binding of FYN SH3 and LCK SH3 with the SK and SK4 regions of SKAP55. SKAP55 constructs were expressed in T cells and subjected to protein–protein blotting with FYN SH3 (lanes 1–8) and LCK SH3 (lanes 9–16). Detection was conducted using anti-Flag antibody coupled to alkaline phosphatase. Lane 1, pGEX-SKAP55(1–106); lane 2, pGEX-SKAP55(105–208); lane 3, pGEX-SKAP55(204–299); lane 4, pGEX-SKAP55(300–369); lane 5, pGEX-SK1(204–238); lane 6, pGEX-SK2(239–255); lane 7, pGEX-SK3(256–276); lane 8, pGEX-SK4(277–298); lane 9, pGEX-SKAP55(1–106); lane 10, pGEX-SKAP55(105–208); lane 11, pGEX-SKAP55(204–299); lane 12, pGEX-SKAP55(300–369); lane 13, pGEX-SK1(204–238); lane 14, pGEX-SK2(239–255); lane 15, pGEX-SK3(256–276); lane 16, pGEX-SK4(277–298).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.