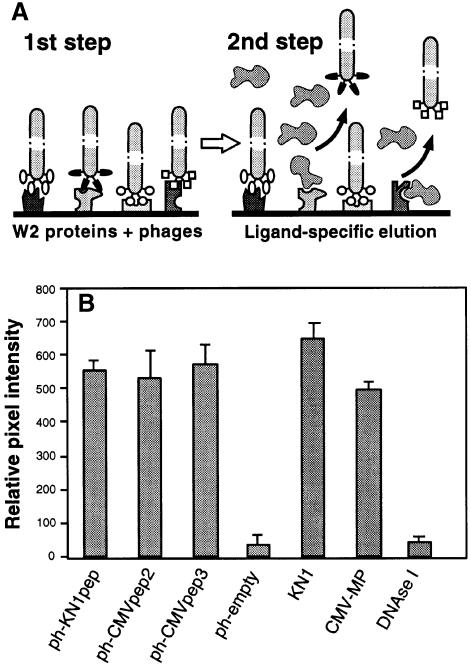
Fig. 2. Isolation and characterization of phages displaying random 12mer peptides that interact with proteins contained within a plasmodesmal-enriched W2 cell fraction. (A) Schematic representation of the protocol employed to screen for peptides capable of interacting with putative plasmodesmal receptors contained within the W2 cell fraction prepared from N.tabacum (tobacco) plants. Immobilized W2 proteins were first incubated with the phage peptide library. After extensive washing to remove unbound phages, ligand competition was carried out to displace a specific subset of the bound phages (ligand-specific elution). Note that non-specific binding of this ligand, to W2 protein-bound phages, would not cause their release. (B) Binding activity between isolated phage-displayed peptides and W2 fraction proteins. Phage probes were first coated onto 6 nm gold particles (ph-KN1pep, ph-CMVpep2, ph-CMVpep3 and ph-empty) and then used for in vitro binding assays to test for their interaction with putative plasmodesmal proteins contained in the W2 cell fraction. KN1-gold, CMV-MP-gold and DNase I-gold probes were employed as controls. Extracted W2 fraction protein (100 ng) was probed with 200 ng of phage-gold, KN1-gold, CMV-MP-gold or DNase I-gold complexes. Interaction of protein-gold probes was visualized by silver enhancement. Data were compiled from three to four replicates, for each experiment, and represent relative pixel densities [after subtraction of background as measured with BSA-gold (see Kragler et al., 1998)]; mean ± SEM.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.