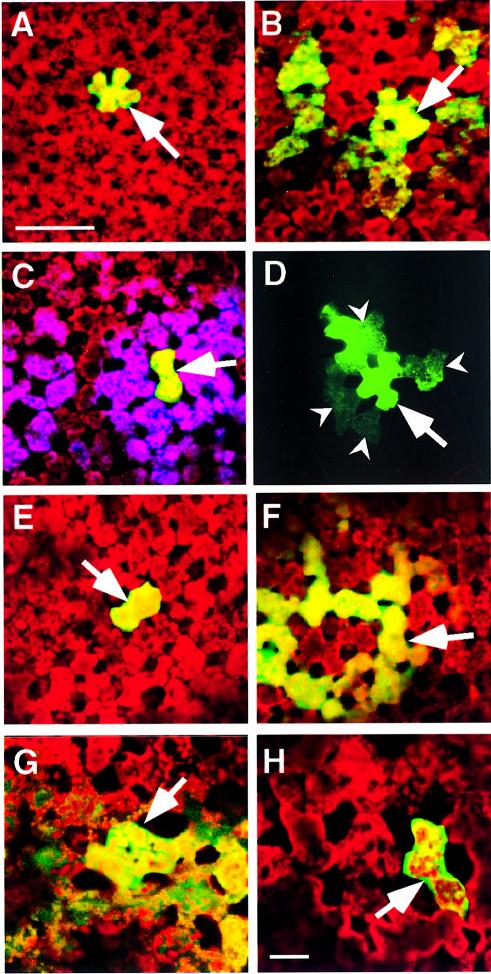
Fig. 4. Phage-displayed peptides and synthetic peptides act as antagonists to KN1-induced increase in plasmodesmal SEL and cell-to-cell movement of macromolecules. All microinjection experiments were carried out on mesophyll cells located within fully expanded leaves of N.benthamiana. A Leica confocal laser scanning microscope was used to simultaneously collect fluorescent signals in the FITC (green) and chlorophyll (red) channels, 10 min after probes were delivered into a target cell. Optical sections, collected in each channel, were stacked and then combined to generate the images presented. (A) Coinjection of ph-KN1pep, KN1 and 11 kDa F-dextran; fluorescent signal (F-dextran) was confined to the target mesophyll cell. (B) Coinjection of ph-empty, KN1 and 11 kDa F-dextran; note that the green fluorescent signal moved from the target cell into neighboring mesophyll cells. (C) Double microinjection experiment; first injection as in (A), in the second injection, Lucifer yellow CH (purple false color) was delivered into the same target cell and moved into the adjacent cells and beyond. (D) KN1-FITC and ph-KN1pep coinjected into a target cell; fluorescent signal associated with KN1 was detected in adjacent cells (darts). Red autofluorescence has been omitted from this image to enhance the visualization of the KN1-FITC signal. (E) Coinjection of KN1pepsynth, KN1 and 11 kDa F-dextran; fluorescent signal (F-dextran) was confined to the target mesophyll cell. (F) Coinjection of CMVpep3synth, KN1 and 11 kDa F-dextran; fluorescent signal (F-dextran) detected in the surrounding mesophyll cells. (G) KN1-mediated transport of KN1–sense RNA-CF. (H) KN1-mediated transport of KN1–sense RNA-CF is blocked in the presence of KN1pepsynth. Arrows indicate the injected cells; scale bar in (A) = 100 µm, and is common to (B)–(F); scale bar in (H) = 50 µm, and is common to (G).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.