Abstract
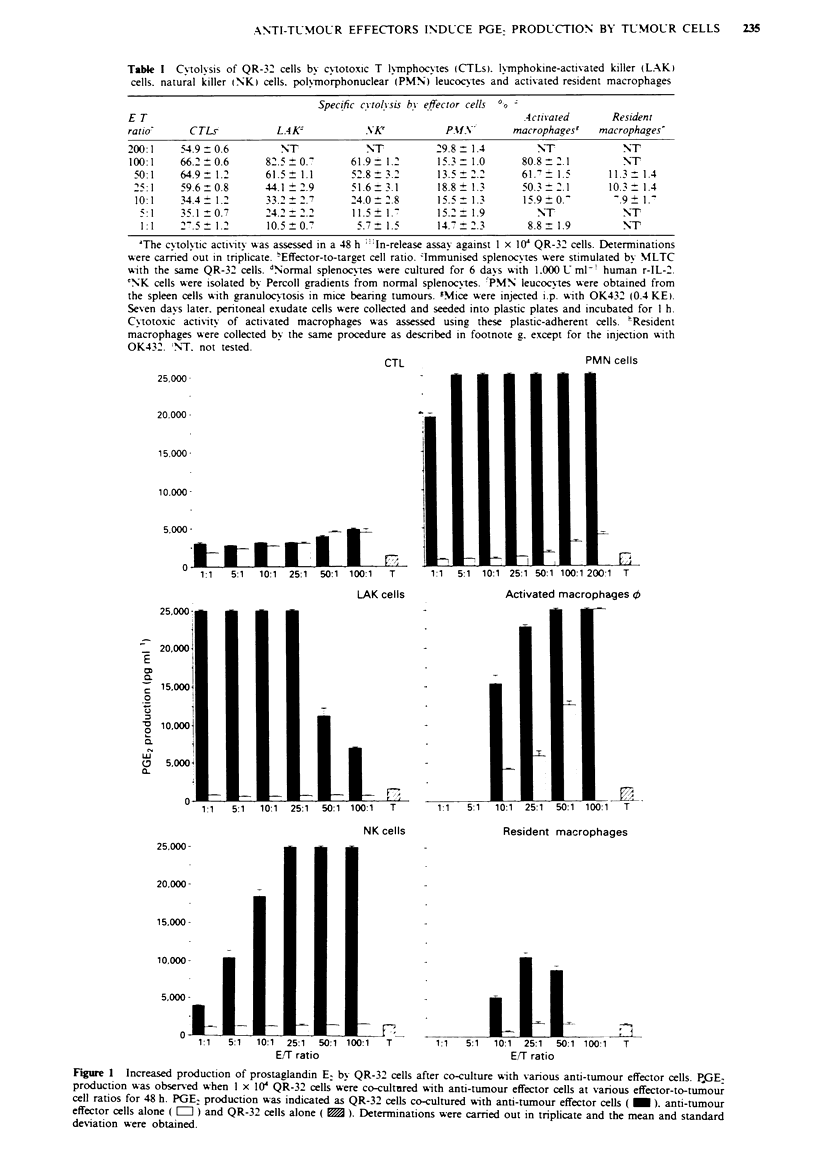
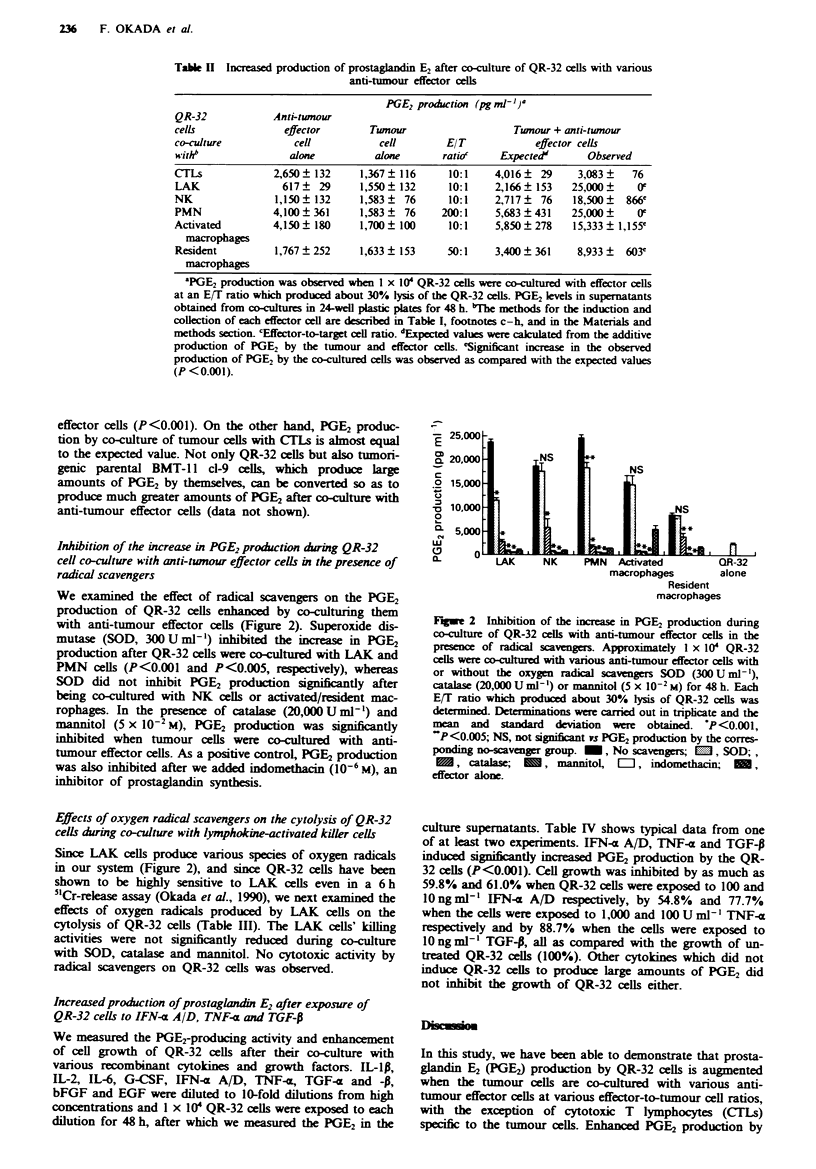
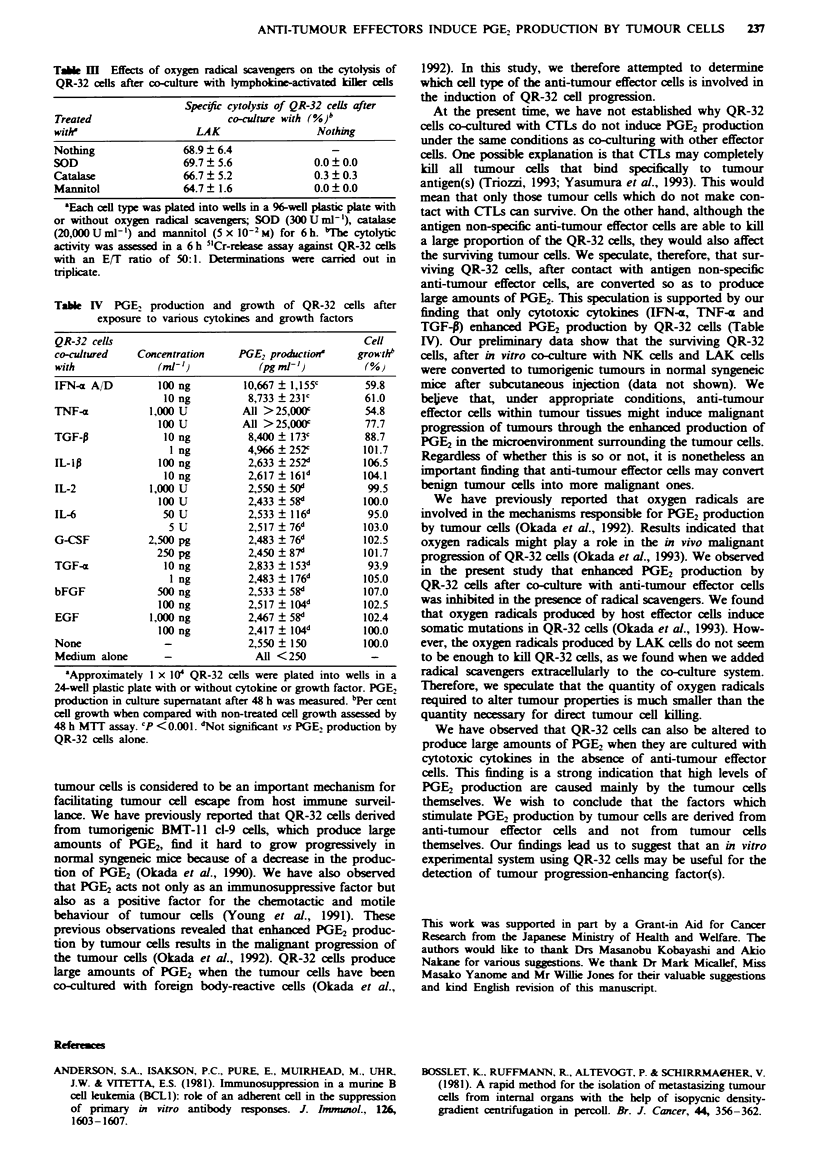
We have previously reported that an increase in the production of immunosuppressive prostaglandin E2 by a QR tumour (QR-32) is accompanied by progressive growth of the tumour in syngeneic C57BL/6 mice. In order to determine what kinds of cell and factor(s) enable QR-32 cells to promote PGE2 production, we investigated the amounts of PGE2 in the supernatant of QR-32 cells by co-culturing them with various anti-tumour effector cells. Significantly high levels of PGE2 production were observed when the QR-32 cells were co-cultured with lymphokine-activated killer (LAK) cells, natural killer (NK) cells, polymorphonuclear (PMN) leucocytes and streptococcal preparation (OK432)-activated or resident peritoneal macrophages (activated and resident macrophages). On the other hand, PGE2 production was not increased when QR-32 cells were co-cultured with cytotoxic T lymphocytes (CTLs) specific to QR-32 cells. The high levels of PGE2 production were partially or totally inhibited by the presence of radical scavengers such as superoxide dismutase (SOD), catalase and mannitol, although the cytotoxicity of LAK cells was not. We also exposed QR-32 cells to human recombinant cytokines and the growth factors which are produced when anti-tumour effector cells come in contact with tumour cells. Significant PGE2 production by QR-32 cells was observed when the cells were treated with interferon alpha (IFN-alpha), tumour necrosis factor alpha (TNF-alpha) and transforming growth factor beta (TGF-beta) (all P < 0.001). These results suggest that oxygen radicals produced by anti-tumour effector cells and inflammatory cytokines provoke QR-32 cells to produce large amounts of immunosuppressive PGE2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. A., Isakson P. C., Puré E., Muirhead M., Uhr J. W., Vitetta E. S. Immunosuppression in a murine B cell leukemia (BCL1): role of an adherent cell in the suppression of primary in vitro antibody responses. J Immunol. 1981 Apr;126(4):1603–1607. [PubMed] [Google Scholar]
- Bosslet K., Ruffmann R., Altevogt P., Schirrmacher V. A rapid method for the isolation of metastasizing tumour cells from internal organs with the help of isopycnic density-gradient centrifugation in Percoll. Br J Cancer. 1981 Sep;44(3):356–362. doi: 10.1038/bjc.1981.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalona W. J., Chretien P. B. Abnormalities of quantitative dinitrochlorobenzene sensitization in cancer patients: correlation with tumor stage and histology. Cancer. 1973 Feb;31(2):353–356. doi: 10.1002/1097-0142(197302)31:2<353::aid-cncr2820310213>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hojo H., Hashimoto Y. Cytotoxic cells induced in tumor-bearing rats by a streptococcus preparation (OK-432). Gan. 1981 Oct;72(5):692–699. [PubMed] [Google Scholar]
- Ishikawa M., Okada F., Hamada J., Hosokawa M., Kobayashi H. Changes in the tumorigenic and metastatic properties of tumor cells treated with quercetin or 5-azacytidine. Int J Cancer. 1987 Mar 15;39(3):338–342. doi: 10.1002/ijc.2910390312. [DOI] [PubMed] [Google Scholar]
- Jessup J. M., Cohen M. H., Tomaszewski M. M., Felix E. L. Effects of murine tumors on delayed hypersensitivity to dinitrochlorobenzene. I. Description of anergy caused by transplanted tumors. J Natl Cancer Inst. 1976 Nov;57(5):1077–1084. doi: 10.1093/jnci/57.5.1077. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Suematsu M., Koizumi H. M., Mitsui H., Suzuki S., Matsuno T., Ogawa H., Nomoto K. Activation of macrophage function by intraperitoneal administration of the streptococcal antitumor agent OK-432. Immunopharmacology. 1983 Oct;6(3):177–189. doi: 10.1016/0162-3109(83)90019-x. [DOI] [PubMed] [Google Scholar]
- Kawata A., Hosokawa M., Sawamura Y., Ito K., Une Y., Shibata T., Uchino J., Kobayashi H. Modification of lymphokine-activated killer cell accumulation into tumor sites by chemotherapy, local irradiation, or splenectomy. Mol Biother. 1990 Dec;2(4):221–227. [PubMed] [Google Scholar]
- Lynch N. R., Salomon J. C. Tumor growth inhibition and potentiation of immunotherapy by indomethacin in mice. J Natl Cancer Inst. 1979 Jan;62(1):117–121. [PubMed] [Google Scholar]
- Mizobe F., Martial E., Colby-Germinario S., Livett B. G. An improved technique for the isolation of lymphocytes from small volumes of peripheral mouse blood. J Immunol Methods. 1982;48(3):269–279. doi: 10.1016/0022-1759(82)90327-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Okada F., Hosokawa M., Hamada J. I., Hasegawa J., Kato M., Mizutani M., Ren J., Takeichi N., Kobayashi H. Malignant progression of a mouse fibrosarcoma by host cells reactive to a foreign body (gelatin sponge). Br J Cancer. 1992 Oct;66(4):635–639. doi: 10.1038/bjc.1992.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada F., Hosokawa M., Hasegawa J., Ishikawa M., Chiba I., Nakamura Y., Kobayashi H. Regression mechanisms of mouse fibrosarcoma cells after in vitro exposure to quercetin: diminution of tumorigenicity with a corresponding decrease in the production of prostaglandin E2. Cancer Immunol Immunother. 1990;31(6):358–364. doi: 10.1007/BF01741407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland P. H., Martin P. M., Jacquemier J., Rolland A. M., Toga M. Prostaglandin in human breast cancer: Evidence suggesting that an elevated prostaglandin production is a marker of high metastatic potential for neoplastic cells. J Natl Cancer Inst. 1980 May;64(5):1061–1070. [PubMed] [Google Scholar]
- Triozzi P. L. Identification and activation of tumor-reactive cells for adoptive immunotherapy. Stem Cells. 1993 May;11(3):204–211. doi: 10.1002/stem.5530110307. [DOI] [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- Yasumura S., Hirabayashi H., Schwartz D. R., Toso J. F., Johnson J. T., Herberman R. B., Whiteside T. L. Human cytotoxic T-cell lines with restricted specificity for squamous cell carcinoma of the head and neck. Cancer Res. 1993 Mar 15;53(6):1461–1468. [PubMed] [Google Scholar]
- Young M. R., Dizer M. Enhancement of immune function and tumor growth inhibition by antibodies against prostaglandin E2. Immunol Commun. 1983;12(1):11–23. doi: 10.3109/08820138309060853. [DOI] [PubMed] [Google Scholar]
- Young M. R., Knies S. Prostaglandin E production by Lewis lung carcinoma: mechanism for tumor establishment in vivo. J Natl Cancer Inst. 1984 Apr;72(4):919–922. [PubMed] [Google Scholar]
- Young M. R., Okada F., Tada M., Hosokawa M., Kobayashi H. Association of increased tumor cell responsiveness to prostaglandin E2 with more aggressive tumor behavior. Invasion Metastasis. 1991;11(1):48–57. [PubMed] [Google Scholar]


