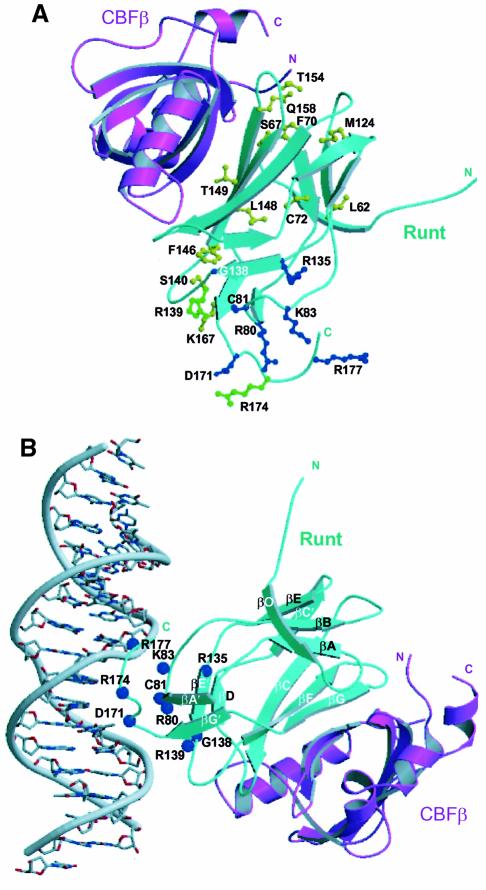
Fig. 7. Location of human disease mutations on the AML1–CBFβ structure. (A) A ribbon diagram of the Runt domain–CBFβ heterodimer in which residues mutated in cleidocranial dysplasia (CCD) are shown in yellow, familial platelet disorder (FPD) and acute myeloid leukaemia (AML) are shown in blue. Mutations common to CCD and FPD/AML are shown in green. The ribbon representation of the Runt domain is in cyan, and that of CBFβ is in magenta. (The AML-associated biallelic point mutations G138D, R135G, D171G are a personal communication from P.Fenaux.) (B) Model orienting the Runt domain–CBFβ heterodimer with respect to B-form DNA. The model was generated by least squares superposition of the co-ordinates of the STAT3β–DNA complex (PDB code, 1bg1) (Becker et al., 1998) onto the AML1 Runt domain. The amino acid residues mutated in human disease mutations are indicated as blue spheres. The Runt domain is shown in cyan, and CBFβ in magenta. The model is consistent with: the electrostatic surface potential of the heterodimer (Figure 4C); in vitro mutagenesis data (Lenny et al., 1995; Kagoshima et al., 1996; Osato et al., 1999); chemical footprinting analysis (Thornell et al., 1988; Melnikova et al., 1993); NOE data from NMR studies (Nagata et al., 1999); and the observations that CBFβ does not contact DNA directly, or extend the Runt domain footprint on DNA (Kamachi et al., 1990).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.