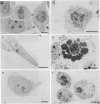
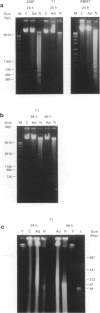
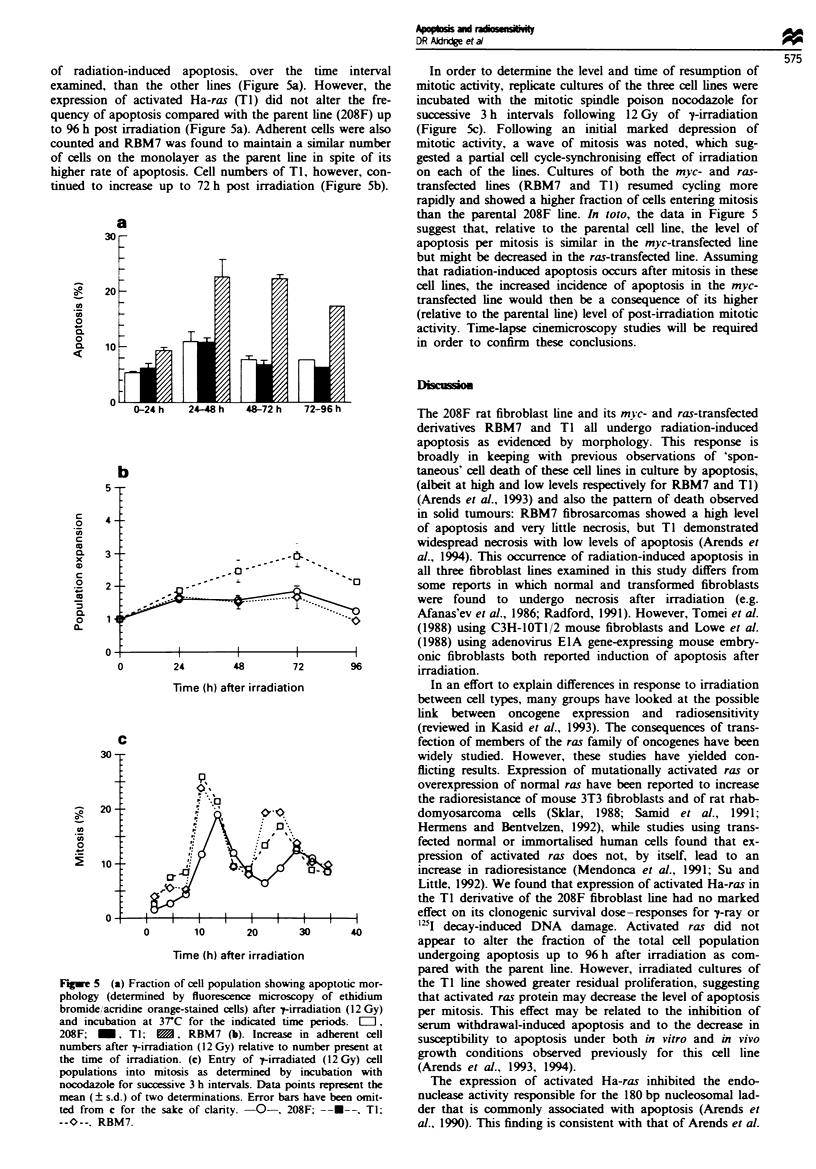
Abstract
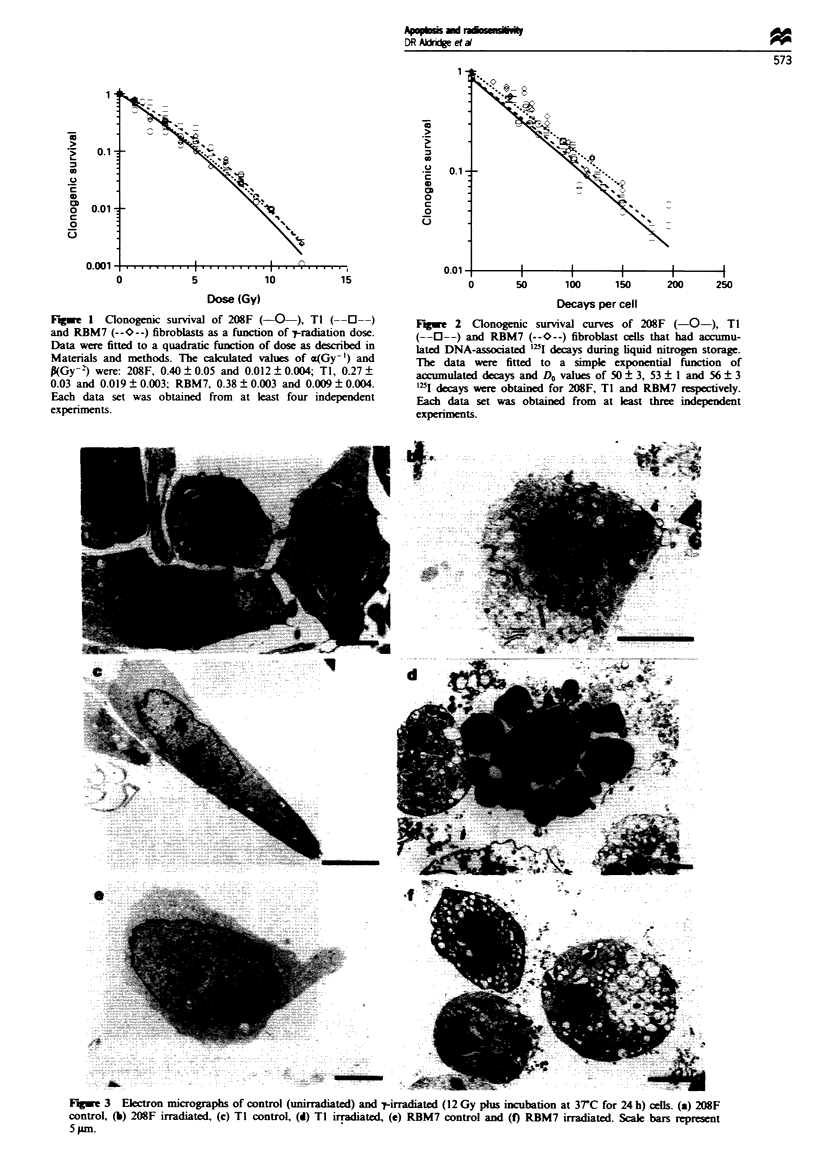
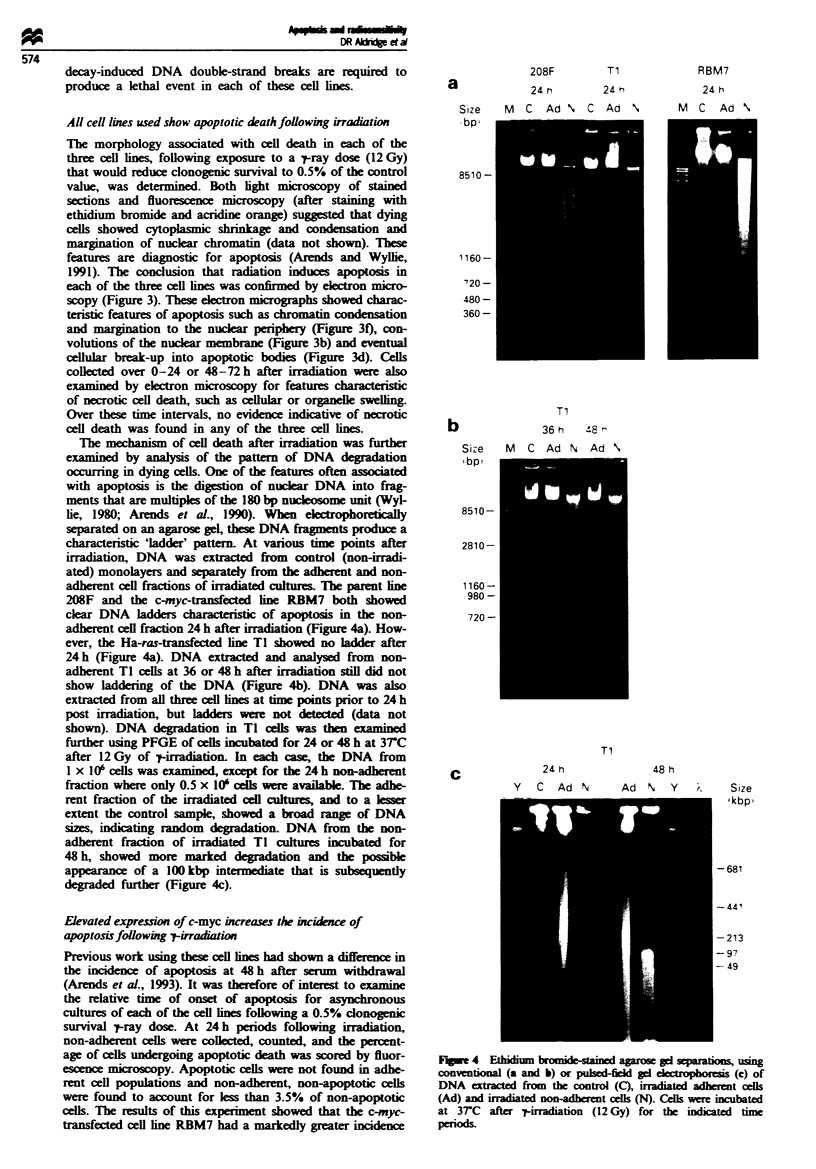
Recent studies have suggested a correlation between the rate and incidence of apoptosis and the radiation response of particular cell lines. However, we found that increasing the rate of induction of apoptosis in the fibroblast line 208F, by transfecting it with human c-myc, did not lead to a change in its clonogenic survival dose-response for either gamma-irradiation or 125I-induced DNA damage. It was also found that expression of mutant (T24) Ha-ras in the 208F line appeared to decrease the level of apoptosis per mitosis after irradiation and inhibited the formation of nucleosomal ladders, but did not affect either the onset of the morphological features of apoptosis or the clonogenic survival dose-response of the cells to either gamma-irradiation or 125I-induced DNA damage. Our findings suggest that it may be incorrect to make predictions about the radiosensitivity of cells based only on knowledge of their mode of death.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afanas'ev V. N., Korol B. A., Mantsygin YuA, Nelipovich P. A., Pechatnikov V. A., Umansky S. R. Flow cytometry and biochemical analysis of DNA degradation characteristic of two types of cell death. FEBS Lett. 1986 Jan 6;194(2):347–350. doi: 10.1016/0014-5793(86)80115-6. [DOI] [PubMed] [Google Scholar]
- Arends M. J., McGregor A. H., Toft N. J., Brown E. J., Wyllie A. H. Susceptibility to apoptosis is differentially regulated by c-myc and mutated Ha-ras oncogenes and is associated with endonuclease availability. Br J Cancer. 1993 Dec;68(6):1127–1133. doi: 10.1038/bjc.1993.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., McGregor A. H., Wyllie A. H. Apoptosis is inversely related to necrosis and determines net growth in tumors bearing constitutively expressed myc, ras, and HPV oncogenes. Am J Pathol. 1994 May;144(5):1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Brown D. G., Sun X. M., Cohen G. M. Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J Biol Chem. 1993 Feb 15;268(5):3037–3039. [PubMed] [Google Scholar]
- Burnet N. G., Nyman J., Turesson I., Wurm R., Yarnold J. R., Peacock J. H. Prediction of normal-tissue tolerance to radiotherapy from in-vitro cellular radiation sensitivity. Lancet. 1992 Jun 27;339(8809):1570–1571. doi: 10.1016/0140-6736(92)91833-t. [DOI] [PubMed] [Google Scholar]
- Charlton D. E. The range of high LET effects from 125I decays. Radiat Res. 1986 Aug;107(2):163–171. [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Snowden R. T., Dinsdale D., Skilleter D. N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992 Sep 1;286(Pt 2):331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry J. H. Survival curves for normal-tissue clonogens: a comparison of assessments using in vitro, transplantation, or in situ techniques. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Jan;47(1):3–16. doi: 10.1080/09553008514550021. [DOI] [PubMed] [Google Scholar]
- Hermens A. F., Bentvelzen P. A. Influence of the H-ras oncogene on radiation responses of a rat rhabdomyosarcoma cell line. Cancer Res. 1992 Jun 1;52(11):3073–3082. [PubMed] [Google Scholar]
- Kasid U., Pirollo K., Dritschilo A., Chang E. Oncogenic basis of radiation resistance. Adv Cancer Res. 1993;61:195–233. doi: 10.1016/s0065-230x(08)60959-8. [DOI] [PubMed] [Google Scholar]
- Krisch R. E., Sauri C. J. Further studies of DNA damage and lethality from the decay of iodine-125 in bacteriophages. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Jun;27(6):553–560. doi: 10.1080/09553007514550581. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Mendonca M. S., Boukamp P., Stanbridge E. J., Redpath J. L. The radiosensitivity of human keratinocytes: influence of activated c-H-ras oncogene expression and tumorigenicity. Int J Radiat Biol. 1991 May;59(5):1195–1206. doi: 10.1080/09553009114551071. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Stephens L. C., Ang K. K., Hunter N. R., Brock W. A., Milas L., Peters L. J. Heterogeneity in the development of apoptosis in irradiated murine tumours of different histologies. Int J Radiat Biol. 1993 Nov;64(5):583–591. doi: 10.1080/09553009314551801. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle L. E., Corcoran L. N., Cocks B. G., Bergemann A. D., Whitley J. C., Finch L. R. Pulsed-field electrophoresis indicates larger-than-expected sizes for mycoplasma genomes. Nucleic Acids Res. 1988 Jul 11;16(13):6015–6025. doi: 10.1093/nar/16.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford I. R. Mouse lymphoma cells that undergo interphase death show markedly increased sensitivity to radiation-induced DNA double-strand breakage as compared with cells that undergo mitotic death. Int J Radiat Biol. 1991 Jun;59(6):1353–1369. doi: 10.1080/09553009114551221. [DOI] [PubMed] [Google Scholar]
- Radford I. R., Murphy T. K. Radiation response of mouse lymphoid and myeloid cell lines. Part III. Different signals can lead to apoptosis and may influence sensitivity to killing by DNA double-strand breakage. Int J Radiat Biol. 1994 Feb;65(2):229–239. doi: 10.1080/09553009414550261. [DOI] [PubMed] [Google Scholar]
- Radford I. R., Murphy T. K., Radley J. M., Ellis S. L. Radiation response of mouse lymphoid and myeloid cell lines. Part II. Apoptotic death is shown by all lines examined. Int J Radiat Biol. 1994 Feb;65(2):217–227. doi: 10.1080/09553009414550251. [DOI] [PubMed] [Google Scholar]
- Radford I. R. Radiation response of mouse lymphoid and myeloid cell lines. Part I. Sensitivity to killing by ionizing radiation, rate of loss of viability, and cell type of origin. Int J Radiat Biol. 1994 Feb;65(2):203–215. doi: 10.1080/09553009414550241. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Radiation biology of malignant melanoma. Acta Radiol Oncol. 1986 Jan-Feb;25(1):1–10. doi: 10.3109/02841868609136368. [DOI] [PubMed] [Google Scholar]
- Samid D., Miller A. C., Rimoldi D., Gafner J., Clark E. P. Increased radiation resistance in transformed and nontransformed cells with elevated ras proto-oncogene expression. Radiat Res. 1991 May;126(2):244–250. [PubMed] [Google Scholar]
- Sklar M. D. The ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation. Science. 1988 Feb 5;239(4840):645–647. doi: 10.1126/science.3277276. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Su L. N., Little J. B. Transformation and radiosensitivity of human diploid skin fibroblasts transfected with activated ras oncogene and SV40 T-antigen. Int J Radiat Biol. 1992 Aug;62(2):201–210. doi: 10.1080/09553009214552021. [DOI] [PubMed] [Google Scholar]
- Tomei L. D., Kanter P., Wenner C. E. Inhibition of radiation-induced apoptosis in vitro by tumor promoters. Biochem Biophys Res Commun. 1988 Aug 30;155(1):324–331. doi: 10.1016/s0006-291x(88)81088-x. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]