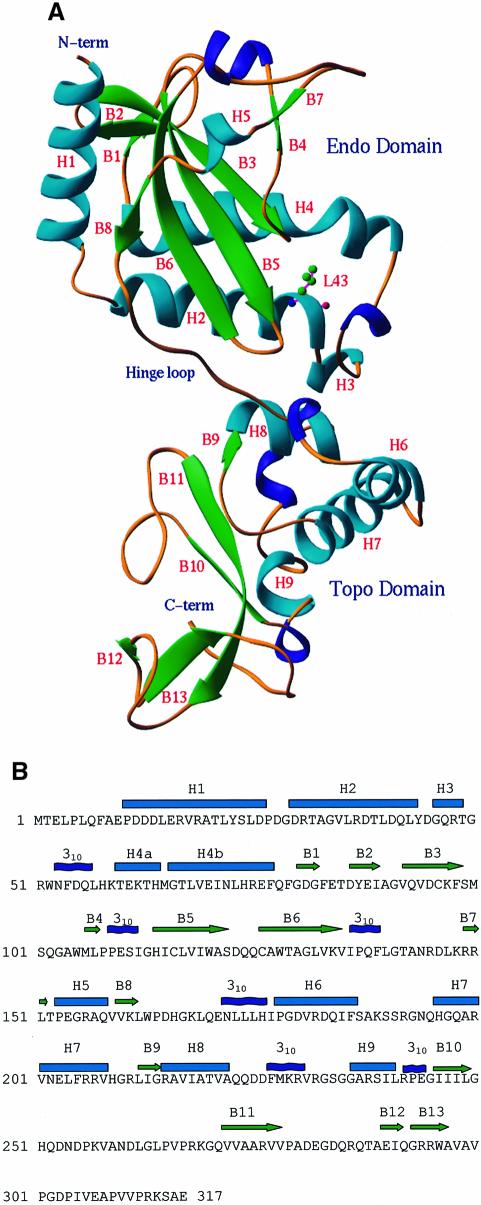
Fig. 1. Structure of the NaeI monomer. (A) Ribbon diagram: α-helices in cyan, 310 helices in blue and β-strands in green. The N-terminal (Endo) domain has topology similar to other restriction endonucleases. The C-terminal (Topo) domain contains a CAP or helix–turn–helix motif (H7, H8, 310 helix before H9, B10, B11) for DNA binding. The hinge loop is around Gln170. L43 is shown in ball-and-stick representation. (B) Sequence and secondary structure of NaeI. Cyan cylinders and green arrows represent α-helices and β-strands, respectively. The helices H4a and H4b are bent at Met65.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.