Abstract
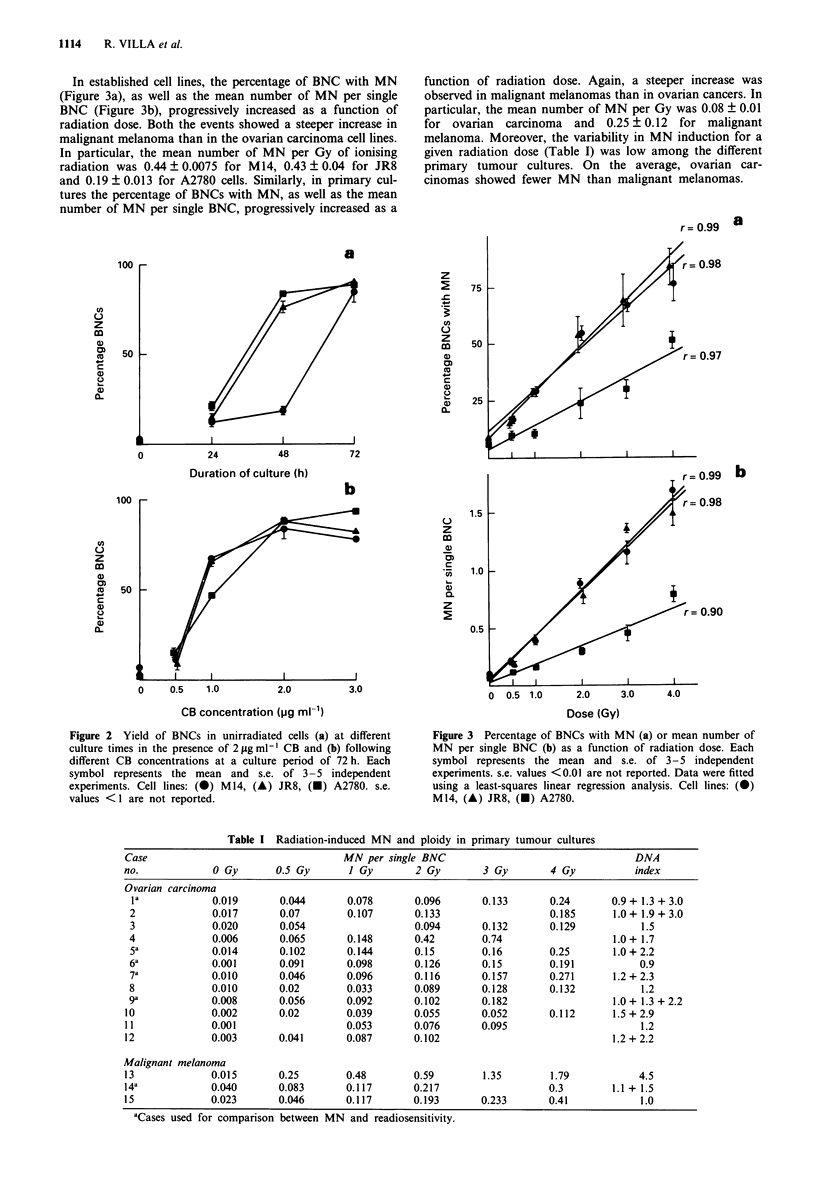
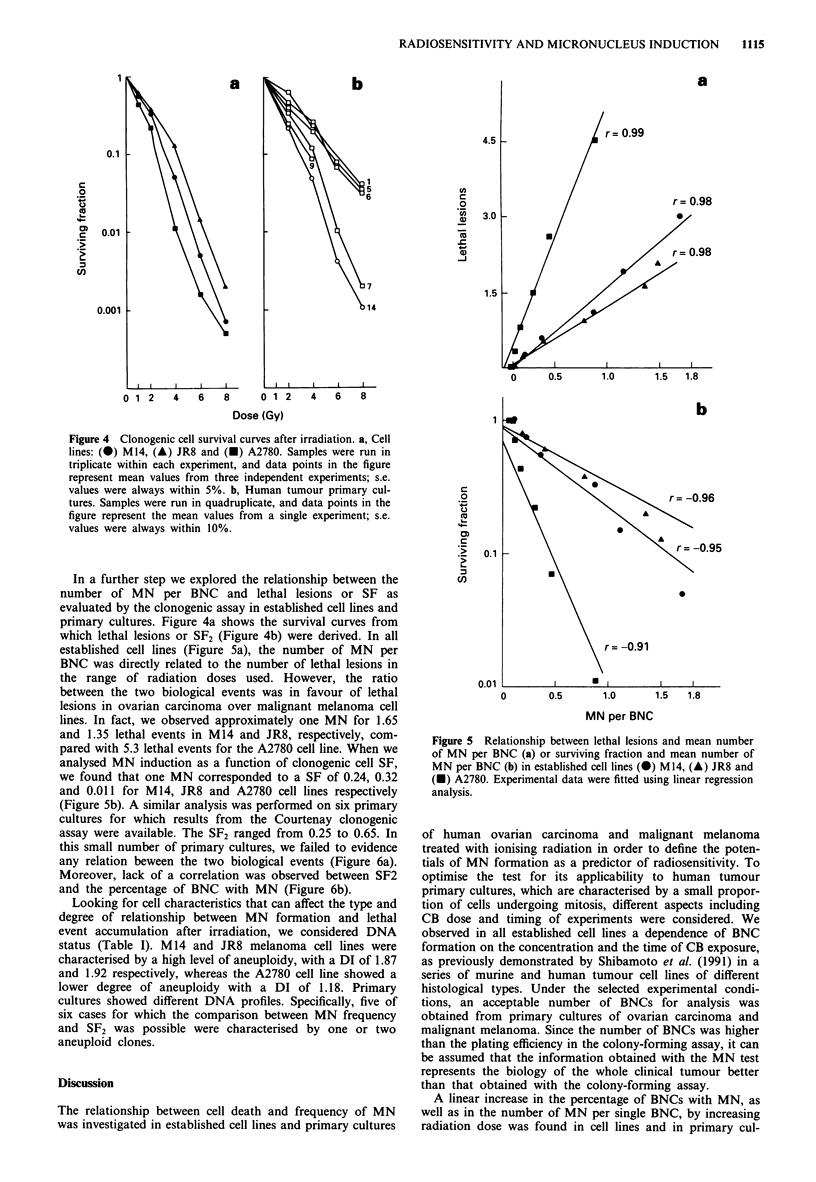
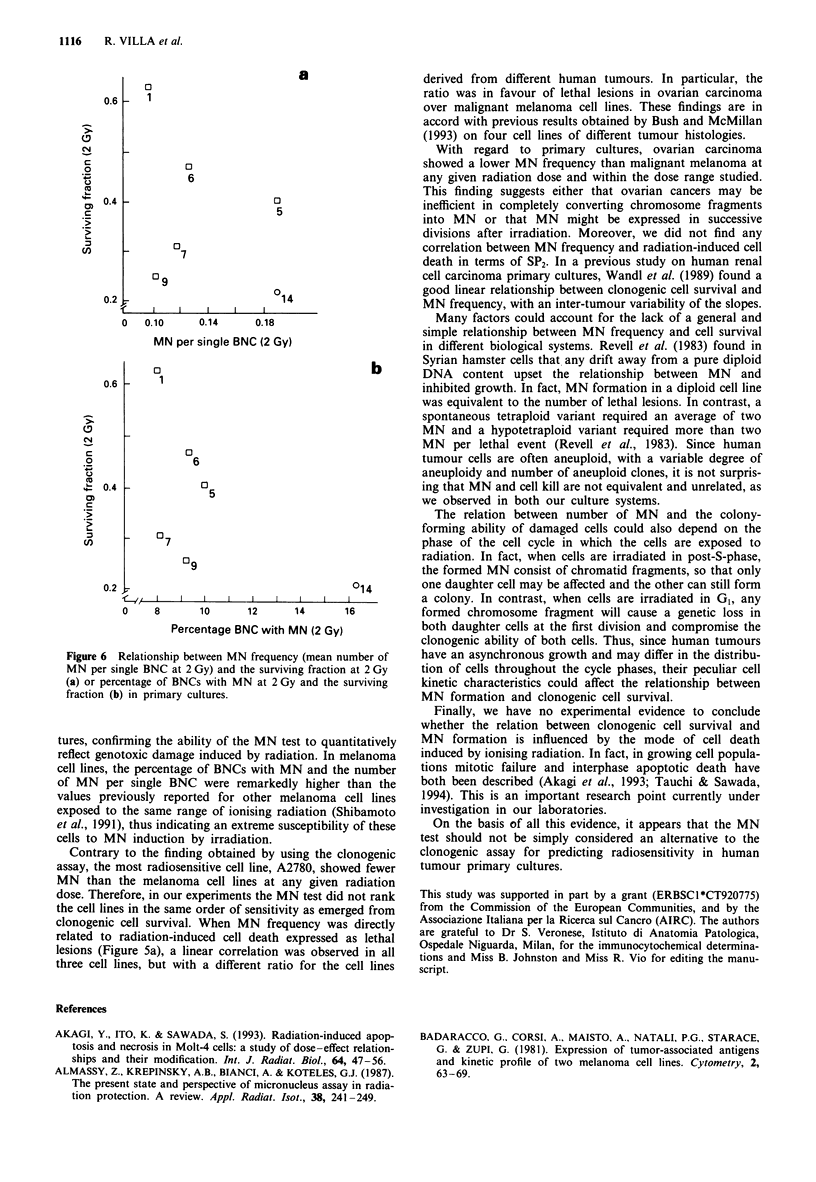
The radiation-induced genotoxic damage in three established cell lines and 15 primary cultures of human malignant melanoma and ovarian carcinoma showing different radiosensitivity was tested by the cytokinesis-block micronucleus assay. A dose-related increase in micronucleus frequency was observed in all the cell systems. The mean number of micronuclei per Gy of ionising radiation per binucleated cell was respectively 0.44 +/- 0.0075 and 0.43 +/- 0.04 for M14 and JR8 malignant melanoma cell lines and 0.19 +/- 0.013 for the A2780 ovarian cancer cell line. The number of micronuclei did not rank the cell lines in the same order of radiosensitivity as clonogenic cell survival, which showed a surviving fraction at 2 Gy of 0.38 +/- 0.02 for JR8, 0.34 +/- 0.05 for M14 and 0.22 +/- 0.007 for A2780. As regards primary tumour cultures, no correlation was observed between micronucleus induction and surviving fraction at 2 Gy. In conclusion, the discrepancy we observed between micronucleus formation and cell death raises doubts about the potential of the micronucleus assay as a preclinical means to predict radiosensitivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi Y., Ito K., Sawada S. Radiation-induced apoptosis and necrosis in Molt-4 cells: a study of dose-effect relationships and their modification. Int J Radiat Biol. 1993 Jul;64(1):47–56. doi: 10.1080/09553009314551101. [DOI] [PubMed] [Google Scholar]
- Almássy Z., Krepinsky A. B., Bianco A., Köteles G. J. The present state and perspectives of micronucleus assay in radiation protection. A review. Int J Rad Appl Instrum A. 1987;38(4):241–249. doi: 10.1016/0883-2889(87)90033-5. [DOI] [PubMed] [Google Scholar]
- Badaracco G., Corsi A., Maisto A., Natali P. G., Starace G., Zupi G. Expression of tumor-associated antigens and kinetic profile of two in vitro human melanoma cell lines. Cytometry. 1981 Sep;2(2):63–69. doi: 10.1002/cyto.990020205. [DOI] [PubMed] [Google Scholar]
- Bakker P. J., Tukker L. J., Stap J., Veenhof C. H., Aten J. A. Micronuclei expression in tumors as a test for radiation sensitivity. Radiother Oncol. 1993 Jan;26(1):69–72. doi: 10.1016/0167-8140(93)90028-7. [DOI] [PubMed] [Google Scholar]
- Bush C., McMillan T. J. Micronucleus formation in human tumour cells: lack of correlation with radiosensitivity. Br J Cancer. 1993 Jan;67(1):102–106. doi: 10.1038/bjc.1993.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Selby P. J., Smith I. E., Mills J., Peckham M. J. Growth of human tumour cell colonies from biopsies using two soft-agar techniques. Br J Cancer. 1978 Jul;38(1):77–81. doi: 10.1038/bjc.1978.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Fenech M., Morley A. Solutions to the kinetic problem in the micronucleus assay. Cytobios. 1985;43(172-173):233–246. [PubMed] [Google Scholar]
- Gantenberg H. W., Wuttke K., Streffer C., Müller W. U. Micronuclei in human lymphocytes irradiated in vitro or in vivo. Radiat Res. 1991 Dec;128(3):276–281. [PubMed] [Google Scholar]
- Heddle J. A., Carrano A. V. The DNA content of micronuclei induced in mouse bone marrow by gamma-irradiation: evidence that micronuclei arise from acentric chromosomal fragments. Mutat Res. 1977 Jul;44(1):63–69. doi: 10.1016/0027-5107(77)90115-4. [DOI] [PubMed] [Google Scholar]
- Heddle J. A., Hite M., Kirkhart B., Mavournin K., MacGregor J. T., Newell G. W., Salamone M. F. The induction of micronuclei as a measure of genotoxicity. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983 Sep;123(1):61–118. doi: 10.1016/0165-1110(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Joshi G. P., Nelson W. J., Revell S. H., Shaw C. A. Discrimination of slow growth from non-survival among small colonies of diploid Syrian hamster cells after chromosome damage induced by a range of x-ray doses. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Sep;42(3):283–296. doi: 10.1080/09553008214551201. [DOI] [PubMed] [Google Scholar]
- Kratochvil M., Rümmelein B., Reimers U., Ehlert U., Weichenthal M., Mensing H., Breitbart E. W., Rüdiger H. W. Constitutively increased micronuclei are predominantly caused by acentric fragments. Mutat Res. 1991 Jul;249(1):223–226. doi: 10.1016/0027-5107(91)90149-i. [DOI] [PubMed] [Google Scholar]
- Meng Z. Q., Zhang L. Z. Observation of frequencies of lymphocytes with micronuclei in human peripheral blood cultures from workers in a sulphuric acid factory. Environ Mol Mutagen. 1990;15(4):218–220. doi: 10.1002/em.2850150408. [DOI] [PubMed] [Google Scholar]
- Midander J., Révész L. The frequency of micronuclei as a measure of cell survival in irradiated cell populations. Int J Radiat Biol Relat Stud Phys Chem Med. 1980 Aug;38(2):237–242. doi: 10.1080/09553008014551161. [DOI] [PubMed] [Google Scholar]
- Migliore L., Parrini M., Sbrana I., Biagini C., Battaglia A., Loprieno N. Micronucleated lymphocytes in people occupationally exposed to potential environmental contaminants: the age effect. Mutat Res. 1991 Jan;256(1):13–20. doi: 10.1016/0921-8734(91)90028-a. [DOI] [PubMed] [Google Scholar]
- Rockwell S. Effects of clumps and clusters on survival measurements with clonogenic assays. Cancer Res. 1985 Apr;45(4):1601–1607. [PubMed] [Google Scholar]
- Rofstad E. K. Radiation response of the cells of a human malignant melanoma xenograft. Effect of hypoxic cell radiosensitizers. Radiat Res. 1981 Sep;87(3):670–683. [PubMed] [Google Scholar]
- Shibamoto Y., Streffer C., Fuhrmann C., Budach V. Tumor radiosensitivity prediction by the cytokinesis-block micronucleus assay. Radiat Res. 1991 Dec;128(3):293–300. [PubMed] [Google Scholar]
- Stap J., Aten J. A. Comparison of radiation sensitivity for three cell lines as measured by the cloning assay and the micro-nucleus test. Strahlenther Onkol. 1990 Nov;166(11):761–763. [PubMed] [Google Scholar]
- Tauchi H., Sawada S. Analysis of mitotic cell death caused by radiation in mouse leukaemia L5178Y cells: apoptosis is the ultimate form of cell death following mitotic failure. Int J Radiat Biol. 1994 Apr;65(4):449–455. doi: 10.1080/09553009414550521. [DOI] [PubMed] [Google Scholar]
- Wandl E. O., Ono K., Kain R., Herbsthofer T., Hienert G., Höbarth K. Linear correlation between surviving fraction and the micronucleus frequency. Int J Radiat Biol. 1989 Nov;56(5):771–775. doi: 10.1080/09553008914552031. [DOI] [PubMed] [Google Scholar]
- West C. M., Davidson S. E., Hunter R. D. Evaluation of surviving fraction at 2 Gy as a potential prognostic factor for the radiotherapy of carcinoma of the cervix. Int J Radiat Biol. 1989 Nov;56(5):761–765. doi: 10.1080/09553008914552011. [DOI] [PubMed] [Google Scholar]
- Yager J. W., Eastmond D. A., Robertson M. L., Paradisin W. M., Smith M. T. Characterization of micronuclei induced in human lymphocytes by benzene metabolites. Cancer Res. 1990 Jan 15;50(2):393–399. [PubMed] [Google Scholar]