Abstract
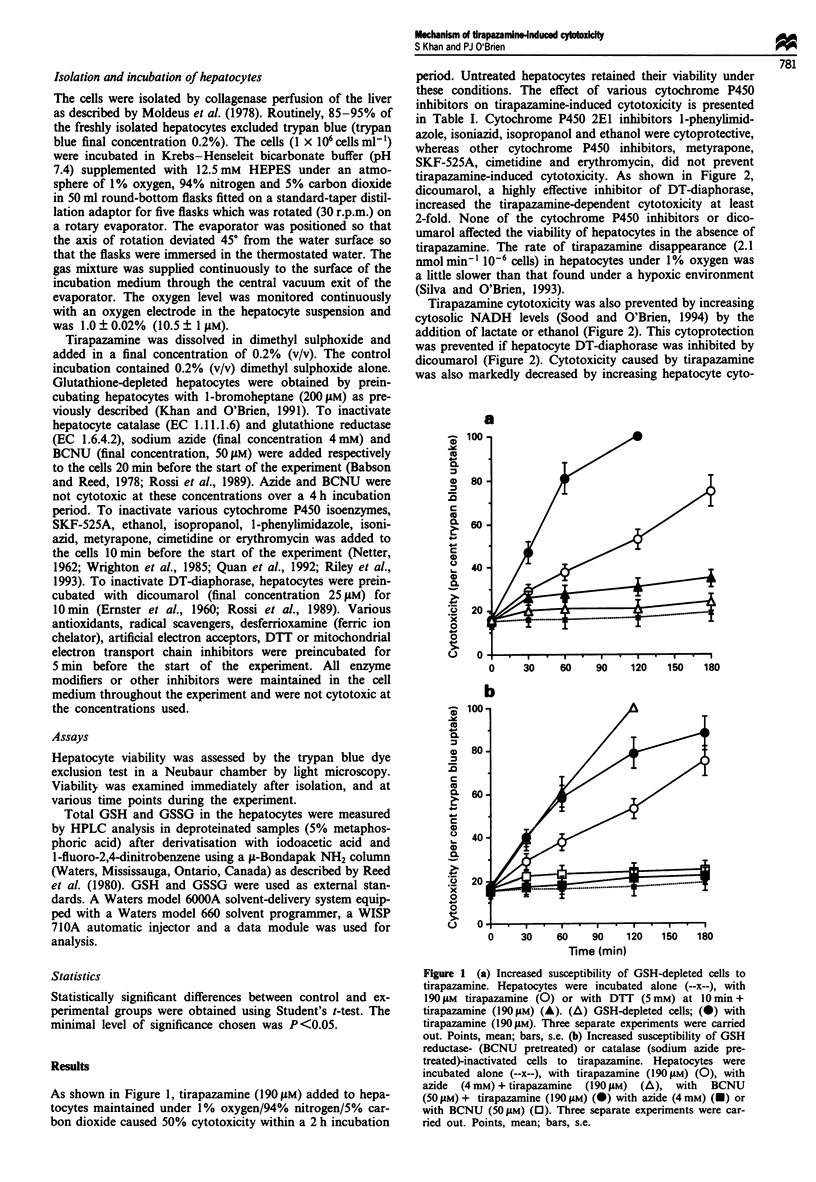
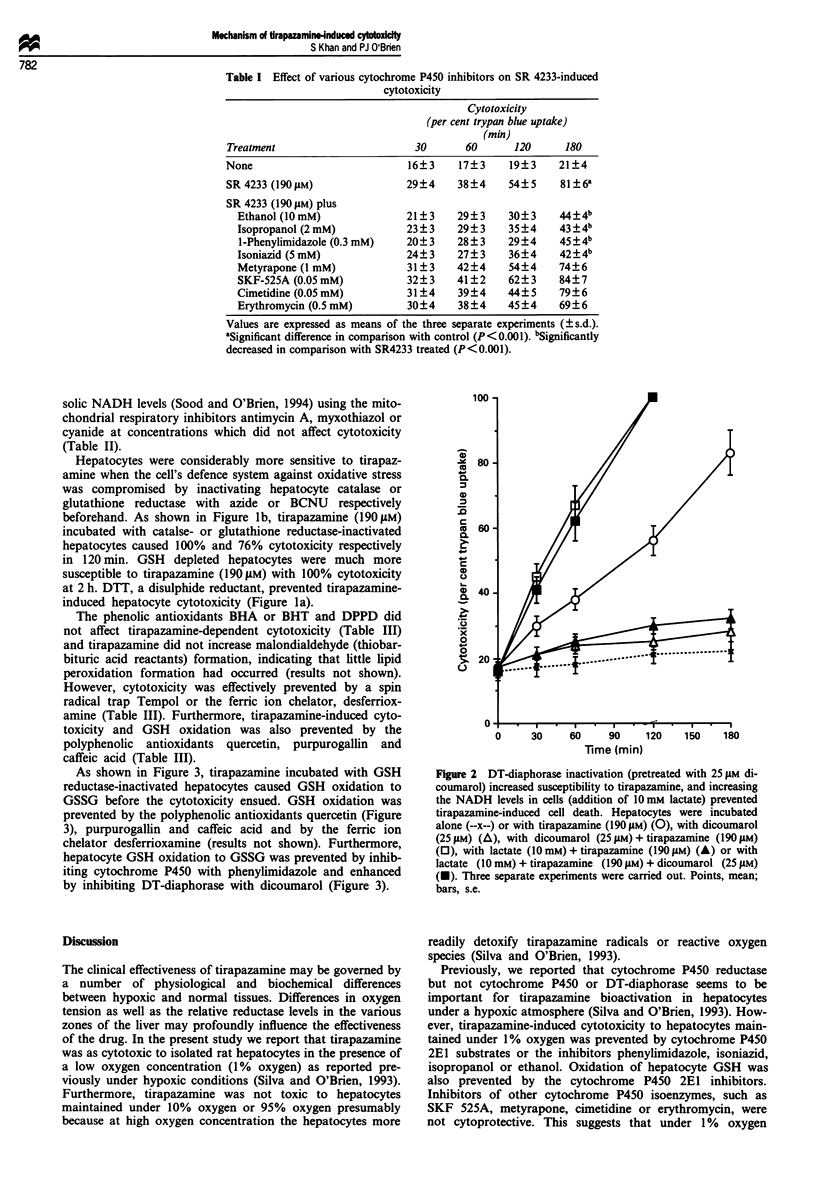
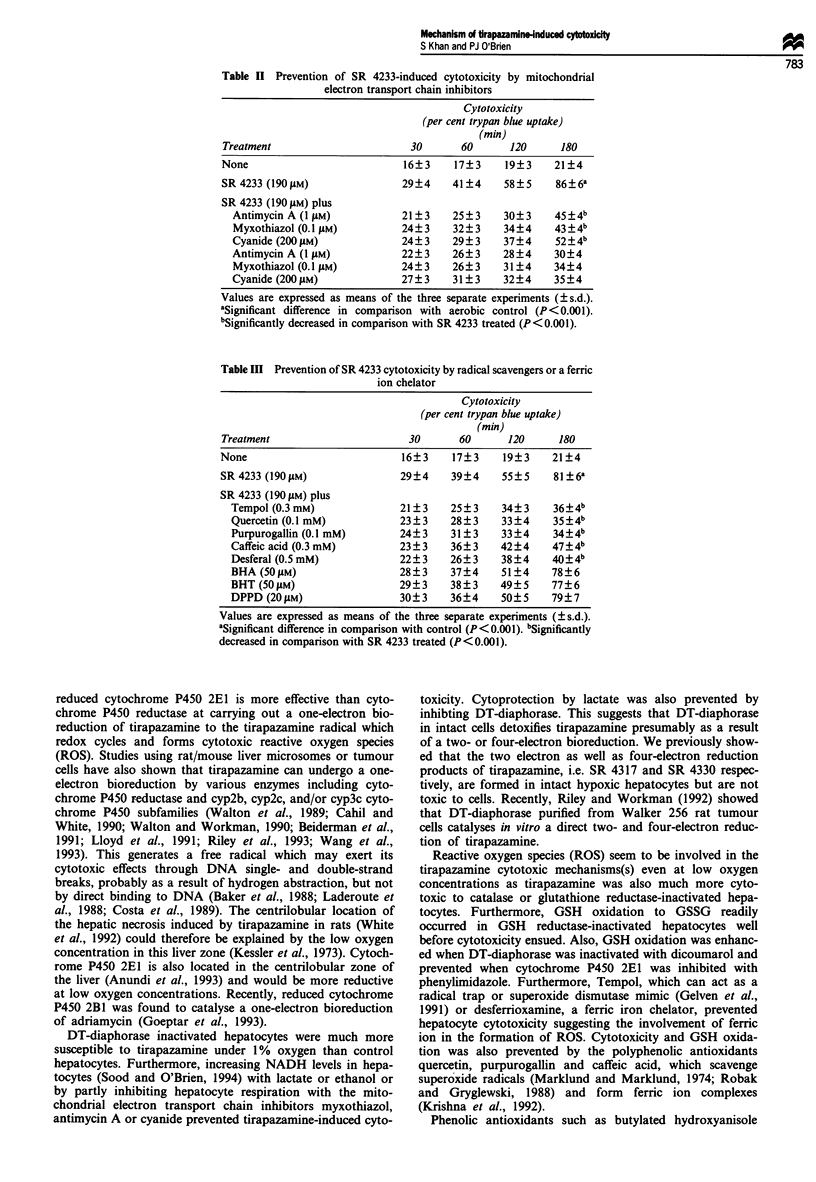
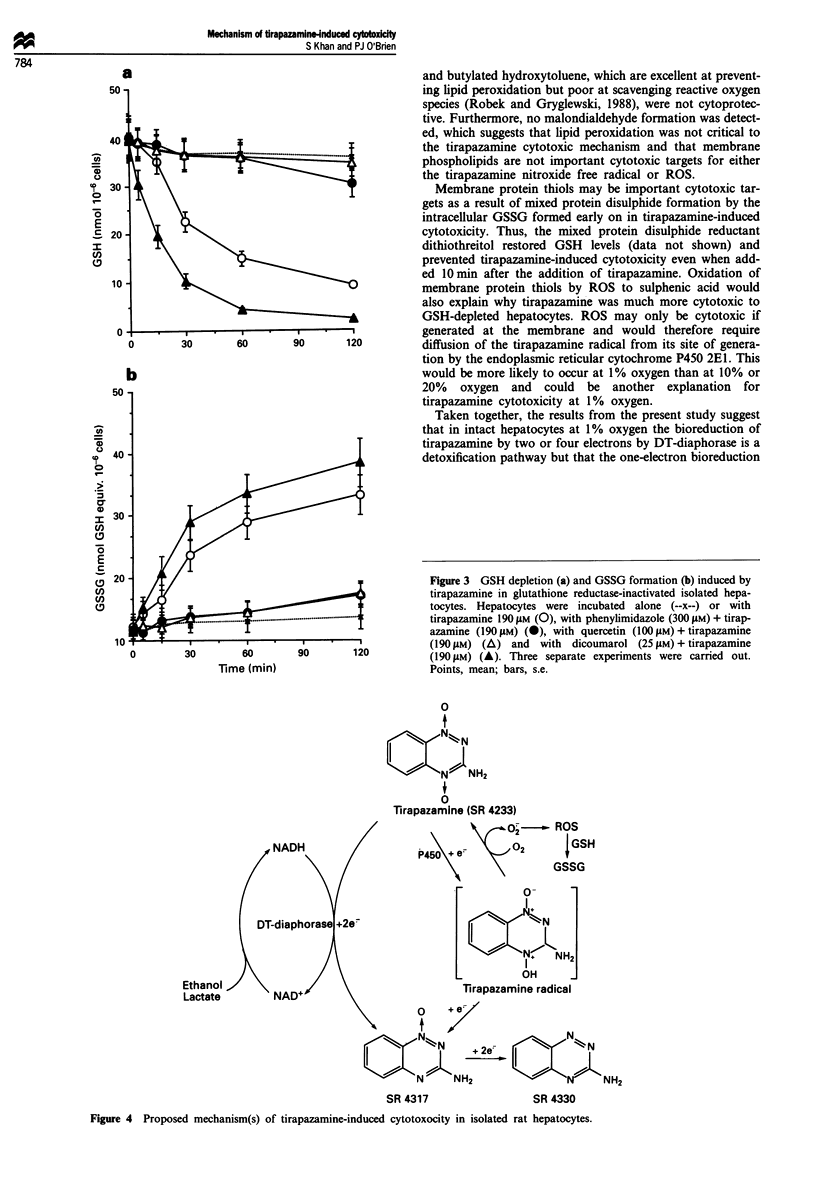
Previously we showed that tirapazamine (SR 4233, Win 59075) is cytotoxic towards hepatocytes under conditions of hypoxia but not in 10% or 95% oxygen and that bioreduction by DT-diaphorase or cytochrome P450 is not a major pathway. In the present study, we report that tirapazamine is highly cytotoxic to isolated rat hepatocytes maintained under 1% oxygen and the molecular cytotoxic mechanism has been elucidated. Cytotoxicity was prevented by the cytochrome P450 2E1 inhibitors phenyl imidazole, isoniazid, isopropanol or ethanol, suggesting that cytochrome P450 2E1 catalysed tirapazamine reductive bioactivation. By contrast, dicoumarol, a DT-diaphorase inhibitor, markedly increased tirapazamine-induced cytotoxicity. Cytotoxicity was also inhibited in normal but not DT-diaphorase-inactivated hepatocytes by increasing cellular NADH levels with lactate or ethanol or the mitochondrial respiratory inhibitors. Evidence that oxygen activation contributed to cytotoxicity was that glutathione oxidation occurred well before cytotoxicity ensued and that tirapazamine was more cytotoxic towards catalase- or glutathione reductase-inactivated hepatocytes. Furthermore, polyphenolic antioxidants such as quercetin, caffeic acid or purpurogallin, the radical trap Tempol or the iron chelator desferrioxamine prevented tirapazamine-mediated cytotoxicity. However, the antioxidants diphenylphenylenediamine, butylated hydroxyanisole or butylated hydroxytoluene did not prevent cytotoxicity and malonaldehyde formation was not increased, suggesting that lipid peroxidation was not important. The above results suggest that DT-diaphorase detoxifies tirapazamine whereas reduced cytochrome P450 reduces tirapazamine to a nitrogen oxide anion radical which forms cytotoxic reactive oxygen species as a result of redox cycling.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anundi I., Lähteenmäki T., Rundgren M., Moldeus P., Lindros K. O. Zonation of acetaminophen metabolism and cytochrome P450 2E1-mediated toxicity studied in isolated periportal and perivenous hepatocytes. Biochem Pharmacol. 1993 Mar 24;45(6):1251–1259. doi: 10.1016/0006-2952(93)90277-4. [DOI] [PubMed] [Google Scholar]
- Babson J. R., Reed D. J. Inactivation of glutathione reductase by 2-chloroethyl nitrosourea-derived isocyanates. Biochem Biophys Res Commun. 1978 Jul 28;83(2):754–762. doi: 10.1016/0006-291x(78)91053-7. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Zeman E. M., Hirst V. K., Brown J. M. Metabolism of SR 4233 by Chinese hamster ovary cells: basis of selective hypoxic cytotoxicity. Cancer Res. 1988 Nov 1;48(21):5947–5952. [PubMed] [Google Scholar]
- Biedermann K. A., Wang J., Graham R. P., Brown J. M. SR 4233 cytotoxicity and metabolism in DNA repair-competent and repair-deficient cell cultures. Br J Cancer. 1991 Mar;63(3):358–362. doi: 10.1038/bjc.1991.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill A., White I. N. Reductive metabolism of 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233) and the induction of unscheduled DNA synthesis in rat and human derived cell lines. Carcinogenesis. 1990 Aug;11(8):1407–1411. doi: 10.1093/carcin/11.8.1407. [DOI] [PubMed] [Google Scholar]
- Costa A. K., Baker M. A., Brown J. M., Trudell J. R. In vitro hepatotoxicity of SR 4233 (3-amino-1,2,4-benzotriazine-1,4-dioxide), a hypoxic cytotoxin and potential antitumor agent. Cancer Res. 1989 Feb 15;49(4):925–929. [PubMed] [Google Scholar]
- Goeptar A. R., Te Koppele J. M., Lamme E. K., Piqué J. M., Vermeulen N. P. Cytochrome P450 2B1-mediated one-electron reduction of adriamycin: a study with rat liver microsomes and purified enzymes. Mol Pharmacol. 1993 Dec;44(6):1267–1277. [PubMed] [Google Scholar]
- Kessler M., Lang H., Sinagowitz E., Rink R., Höper J. Homeostasis of oxygen supply in liver and kidney. Adv Exp Med Biol. 1973;37A:351–360. doi: 10.1007/978-1-4684-3288-6_43. [DOI] [PubMed] [Google Scholar]
- Khan S., O'Brien P. J. 1-bromoalkanes as new potent nontoxic glutathione depletors in isolated rat hepatocytes. Biochem Biophys Res Commun. 1991 Aug 30;179(1):436–441. doi: 10.1016/0006-291x(91)91389-t. [DOI] [PubMed] [Google Scholar]
- Koch C. J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993 Sep 1;53(17):3992–3997. [PubMed] [Google Scholar]
- Krishna C. M., Liebmann J. E., Kaufman D., DeGraff W., Hahn S. M., McMurry T., Mitchell J. B., Russo A. The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch Biochem Biophys. 1992 Apr;294(1):98–106. doi: 10.1016/0003-9861(92)90142-j. [DOI] [PubMed] [Google Scholar]
- Laderoute K., Wardman P., Rauth A. M. Molecular mechanisms for the hypoxia-dependent activation of 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233). Biochem Pharmacol. 1988 Apr 15;37(8):1487–1495. doi: 10.1016/0006-2952(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Duling D. R., Rumyantseva G. V., Mason R. P., Bridson P. K. Microsomal reduction of 3-amino-1,2,4-benzotriazine 1,4-dioxide to a free radical. Mol Pharmacol. 1991 Sep;40(3):440–445. [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Quan Z., Khan S., O'Brien P. J. Role of cytochrome P-450IIE1 in N-nitroso-N-methylaniline induced hepatocyte cytotoxicity. Chem Biol Interact. 1992 Aug 28;83(3):221–233. doi: 10.1016/0009-2797(92)90099-7. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Hemingway S. A., Graham M. A., Workman P. Initial characterization of the major mouse cytochrome P450 enzymes involved in the reductive metabolism of the hypoxic cytotoxin 3-amino-1,2,4-benzotriazine-1,4-di-N-oxide (tirapazamine, SR 4233, WIN 59075). Biochem Pharmacol. 1993 Mar 9;45(5):1065–1077. doi: 10.1016/0006-2952(93)90251-q. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Workman P. Enzymology of the reduction of the potent benzotriazine-di-N-oxide hypoxic cell cytotoxin SR 4233 (WIN 59075) by NAD(P)H: (quinone acceptor) oxidoreductase (EC 1.6.99.2) purified from Walker 256 rat tumour cells. Biochem Pharmacol. 1992 Jan 22;43(2):167–174. doi: 10.1016/0006-2952(92)90274-m. [DOI] [PubMed] [Google Scholar]
- Robak J., Gryglewski R. J. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988 Mar 1;37(5):837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- Silva J. M., O'Brien P. J. Molecular mechanisms of SR 4233-induced hepatocyte toxicity under aerobic versus hypoxic conditions. Br J Cancer. 1993 Sep;68(3):484–491. doi: 10.1038/bjc.1993.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood C., O'Brien P. J. Chloroacetaldehyde-induced hepatocyte cytotoxicity. Mechanisms for cytoprotection. Biochem Pharmacol. 1994 Aug 30;48(5):1025–1032. doi: 10.1016/0006-2952(94)90374-3. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Wolf C. R., Workman P. Molecular enzymology of the reductive bioactivation of hypoxic cell cytotoxins. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):983–986. doi: 10.1016/0360-3016(89)90900-0. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Wolf C. R., Workman P. The role of cytochrome P450 and cytochrome P450 reductase in the reductive bioactivation of the novel benzotriazine di-N-oxide hypoxic cytotoxin 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233, WIN 59075) by mouse liver. Biochem Pharmacol. 1992 Jul 22;44(2):251–259. doi: 10.1016/0006-2952(92)90007-6. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Workman P. Enzymology of the reductive bioactivation of SR 4233. A novel benzotriazine di-N-oxide hypoxic cell cytotoxin. Biochem Pharmacol. 1990 Jun 1;39(11):1735–1742. doi: 10.1016/0006-2952(90)90119-6. [DOI] [PubMed] [Google Scholar]
- Wang J., Biedermann K. A., Wolf C. R., Brown J. M. Metabolism of the bioreductive cytotoxin SR 4233 by tumour cells: enzymatic studies. Br J Cancer. 1993 Feb;67(2):321–325. doi: 10.1038/bjc.1993.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N., Cahill A., Davies A., Carthew P. Acute lesions in rats caused by 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233) or nitromin: a comparison with rates of reduction in microsomal systems from target organs. Arch Toxicol. 1992;66(2):100–106. doi: 10.1007/BF02342502. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Maurel P., Schuetz E. G., Watkins P. B., Young B., Guzelian P. S. Identification of the cytochrome P-450 induced by macrolide antibiotics in rat liver as the glucocorticoid responsive cytochrome P-450p. Biochemistry. 1985 Apr 23;24(9):2171–2178. doi: 10.1021/bi00330a010. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Hirst V. K., Lemmon M. J., Brown J. M. Enhancement of radiation-induced tumor cell killing by the hypoxic cell toxin SR 4233. Radiother Oncol. 1988 Jul;12(3):209–218. doi: 10.1016/0167-8140(88)90263-0. [DOI] [PubMed] [Google Scholar]


