Abstract
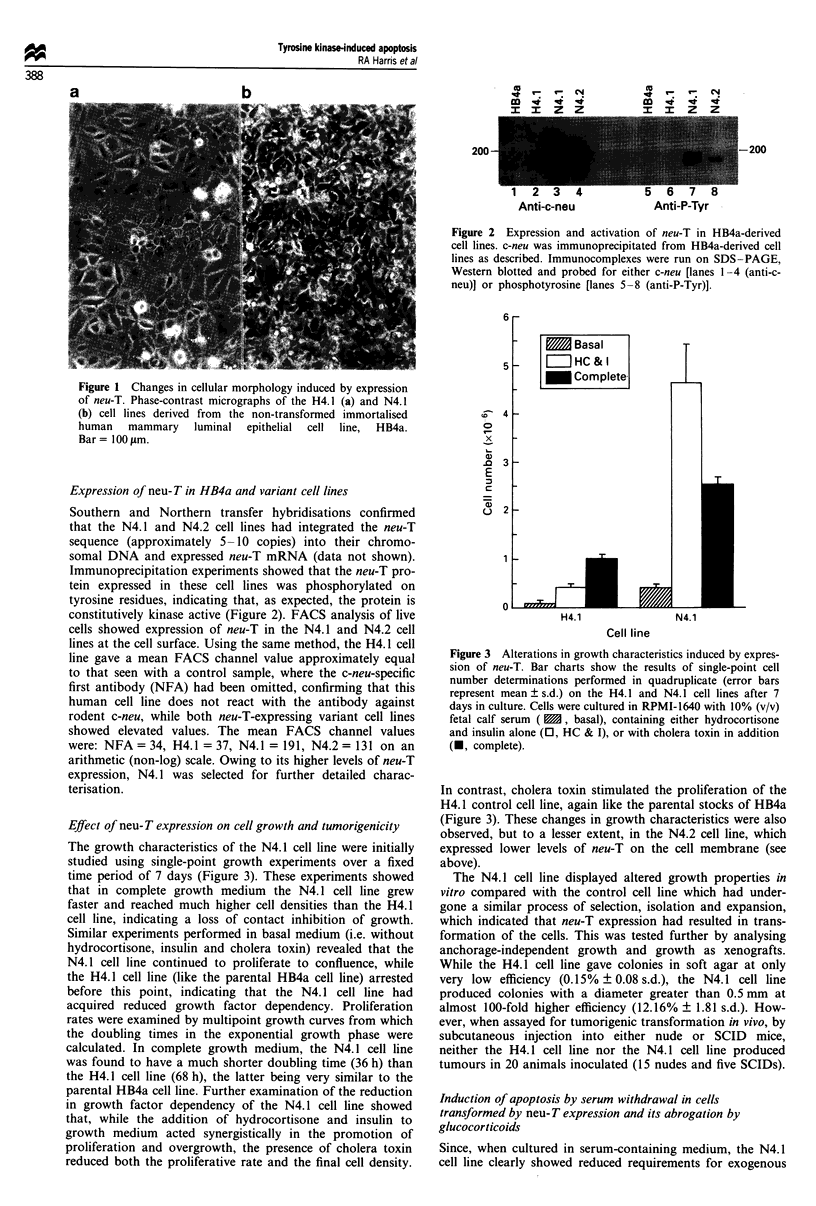
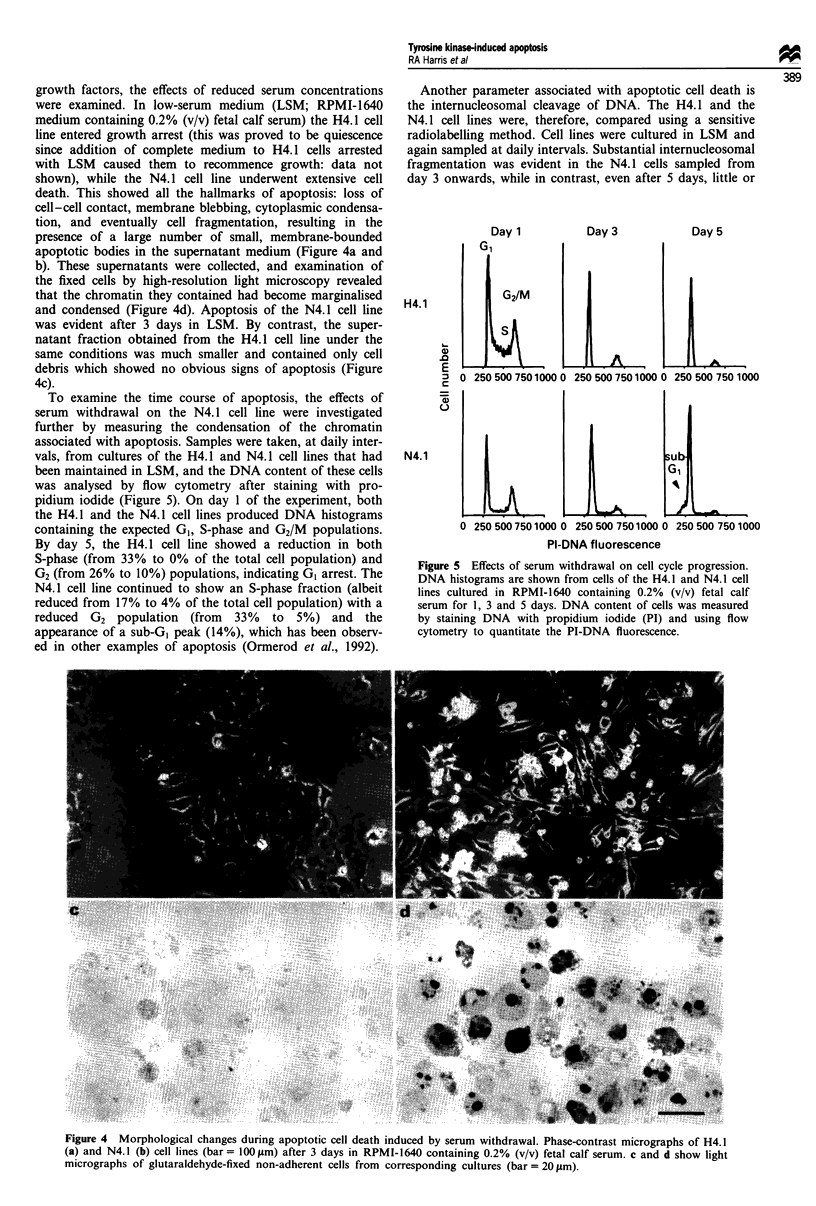
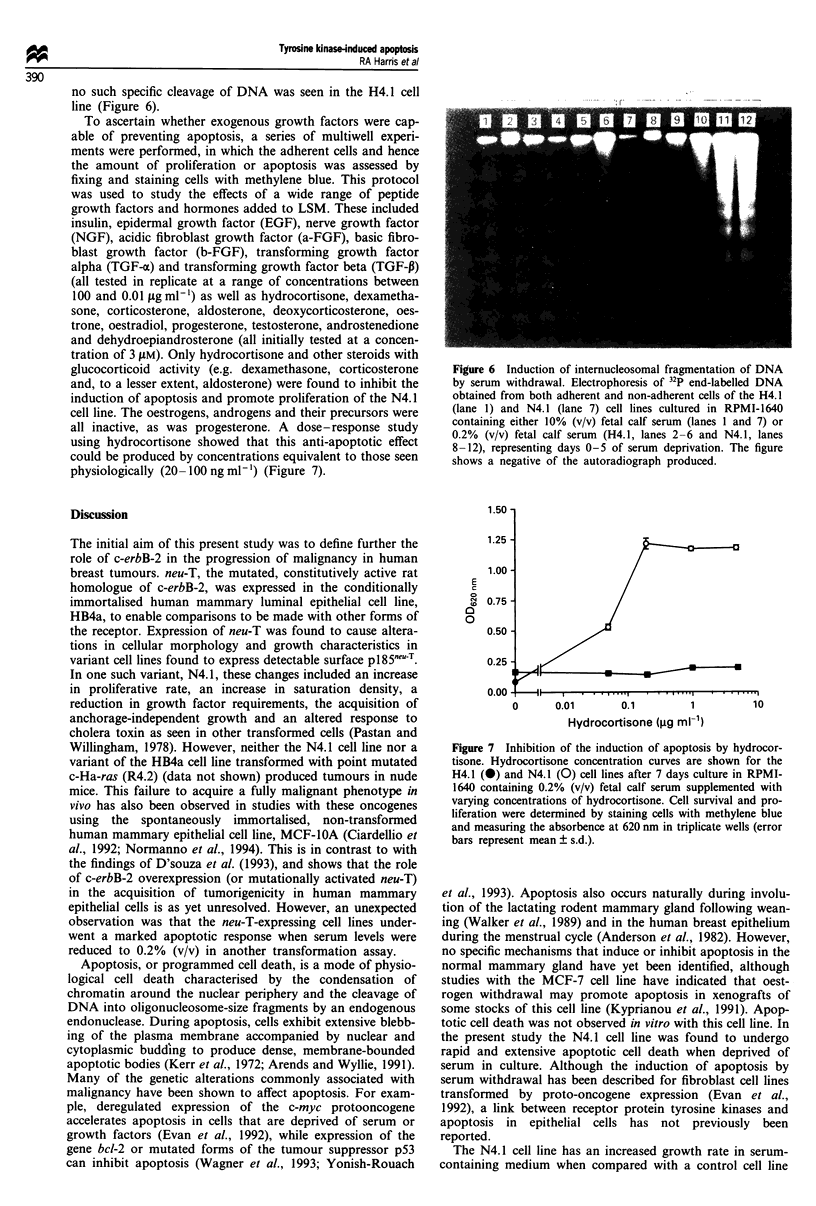
The effects of expressing neu-T, a mutated constitutively activated form of c-neu, have been examined in the non-transformed conditionally immortalised human mammary luminal epithelial cell line, HB4a. A variant cell line, N4.1, which expressed neu-T, showed evidence of transformation, including partial loss of growth factor dependence and acquisition of anchorage-independent growth, but failed to give rise to tumours in nude mice, indicating that expression of neu-T alone was probably insufficient to cause tumorigenic progression to a full malignant phenotype. During characterisation of the N4.1 cell line, it was observed that under conditions of serum deprivation, it underwent apoptotic cell death, as demonstrated by light microscopy, flow cytometry and DNA gel electrophoresis. The induction of apoptotic cell death in the N4.1 cell line by serum deprivation was abrogated specifically by the addition of steroids with glucocorticoid activity but not any peptide growth factors studied. This study shows the induction of apoptosis by serum deprivation, and its abrogation by glucocorticoids occurring in human mammary luminal epithelial cells transformed by expression of neu-T, and implicates the involvement of receptor protein tyrosine kinases in an apoptotic signalling pathway in this cell type.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. J., Ferguson D. J., Raab G. M. Cell turnover in the "resting" human breast: influence of parity, contraceptive pill, age and laterality. Br J Cancer. 1982 Sep;46(3):376–382. doi: 10.1038/bjc.1982.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Ben-Levy R., Paterson H. F., Marshall C. J., Yarden Y. A single autophosphorylation site confers oncogenicity to the Neu/ErbB-2 receptor and enables coupling to the MAP kinase pathway. EMBO J. 1994 Jul 15;13(14):3302–3311. doi: 10.1002/j.1460-2075.1994.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard L., Lamarre L., Tremblay P. J., Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989 Jun 16;57(6):931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- Catchpoole D. R., Stewart B. W. Etoposide-induced cytotoxicity in two human T-cell leukemic lines: delayed loss of membrane permeability rather than DNA fragmentation as an indicator of programmed cell death. Cancer Res. 1993 Sep 15;53(18):4287–4296. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciardiello F., Gottardis M., Basolo F., Pepe S., Normanno N., Dickson R. B., Bianco A. R., Salomon D. S. Additive effects of c-erbB-2, c-Ha-ras, and transforming growth factor-alpha genes on in vitro transformation of human mammary epithelial cells. Mol Carcinog. 1992;6(1):43–52. doi: 10.1002/mc.2940060108. [DOI] [PubMed] [Google Scholar]
- D'Souza B., Berdichevsky F., Kyprianou N., Taylor-Papadimitriou J. Collagen-induced morphogenesis and expression of the alpha 2-integrin subunit is inhibited in c-erbB2-transfected human mammary epithelial cells. Oncogene. 1993 Jul;8(7):1797–1806. [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Kraus M. H., Segatto O., King C. R., Aaronson S. A. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987 Jul 10;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Littlewood T. D. The role of c-myc in cell growth. Curr Opin Genet Dev. 1993 Feb;3(1):44–49. doi: 10.1016/s0959-437x(05)80339-9. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Ewing T. M., Murphy L. J., Ng M. L., Pang G. Y., Lee C. S., Watts C. K., Sutherland R. L. Regulation of epidermal growth factor receptor by progestins and glucocorticoids in human breast cancer cell lines. Int J Cancer. 1989 Oct 15;44(4):744–752. doi: 10.1002/ijc.2910440432. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Love S. B., Wright C., Barnes D. M., Gusterson B., Harris A. L., Altman D. G. c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br J Cancer. 1991 Mar;63(3):434–438. doi: 10.1038/bjc.1991.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington E. A., Bennett M. R., Fanidi A., Evan G. I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994 Jul 15;13(14):3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hudziak R. M., Schlessinger J., Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlan B. Y., Jones J., Slamon D. J., Lagasse L. D. Glucocorticoids stabilize HER-2/neu messenger RNA in human epithelial ovarian carcinoma cells. Gynecol Oncol. 1994 Apr;53(1):70–77. doi: 10.1006/gyno.1994.1090. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Muller W. J., Sinn E., Pattengale P. K., Wallace R., Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988 Jul 1;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Normanno N., Selvam M. P., Qi C. F., Saeki T., Johnson G., Kim N., Ciardiello F., Shoyab M., Plowman G., Brandt R. Amphiregulin as an autocrine growth factor for c-Ha-ras- and c-erbB-2-transformed human mammary epithelial cells. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2790–2794. doi: 10.1073/pnas.91.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare M. J., Ormerod M. G., Monaghan P., Lane E. B., Gusterson B. A. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation. 1991 Apr;46(3):209–221. doi: 10.1111/j.1432-0436.1991.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Ormerod M. G., Collins M. K., Rodriguez-Tarduchy G., Robertson D. Apoptosis in interleukin-3-dependent haemopoietic cells. Quantification by two flow cytometric methods. J Immunol Methods. 1992 Aug 30;153(1-2):57–65. doi: 10.1016/0022-1759(92)90305-d. [DOI] [PubMed] [Google Scholar]
- Ormerod M. G., O'Neill C. F., Robertson D., Harrap K. R. Cisplatin induces apoptosis in a human ovarian carcinoma cell line without concomitant internucleosomal degradation of DNA. Exp Cell Res. 1994 Apr;211(2):231–237. doi: 10.1006/excr.1994.1082. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Biegel D., Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979 Apr;16(4):929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Arnstein P., DiMarco E., Artrip J., Kraus M. H., Lonardo F., Di Fiore P. P., Aaronson S. A. Oncogenic potential of erbB-2 in human mammary epithelial cells. Oncogene. 1991 Jul;6(7):1189–1194. [PubMed] [Google Scholar]
- Rösl F. A simple and rapid method for detection of apoptosis in human cells. Nucleic Acids Res. 1992 Oct 11;20(19):5243–5243. doi: 10.1093/nar/20.19.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Segawa K., Minowa A., Sugasawa K., Takano T., Hanaoka F. Abrogation of p53-mediated transactivation by SV40 large T antigen. Oncogene. 1993 Mar;8(3):543–548. [PubMed] [Google Scholar]
- Stamps A. C., Davies S. C., Burman J., O'Hare M. J. Analysis of proviral integration in human mammary epithelial cell lines immortalized by retroviral infection with a temperature-sensitive SV40 T-antigen construct. Int J Cancer. 1994 Jun 15;57(6):865–874. doi: 10.1002/ijc.2910570616. [DOI] [PubMed] [Google Scholar]
- Topley P., Jenkins D. C., Jessup E. A., Stables J. N. Effect of reconstituted basement membrane components on the growth of a panel of human tumour cell lines in nude mice. Br J Cancer. 1993 May;67(5):953–958. doi: 10.1038/bjc.1993.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wagner A. J., Small M. B., Hay N. Myc-mediated apoptosis is blocked by ectopic expression of Bcl-2. Mol Cell Biol. 1993 Apr;13(4):2432–2440. doi: 10.1128/mcb.13.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N. I., Bennett R. E., Kerr J. F. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989 May;185(1):19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ikawa S., Akiyama T., Semba K., Nomura N., Miyajima N., Saito T., Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986 Jan 16;319(6050):230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Grunwald D., Wilder S., Kimchi A., May E., Lawrence J. J., May P., Oren M. p53-mediated cell death: relationship to cell cycle control. Mol Cell Biol. 1993 Mar;13(3):1415–1423. doi: 10.1128/mcb.13.3.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]






