Abstract
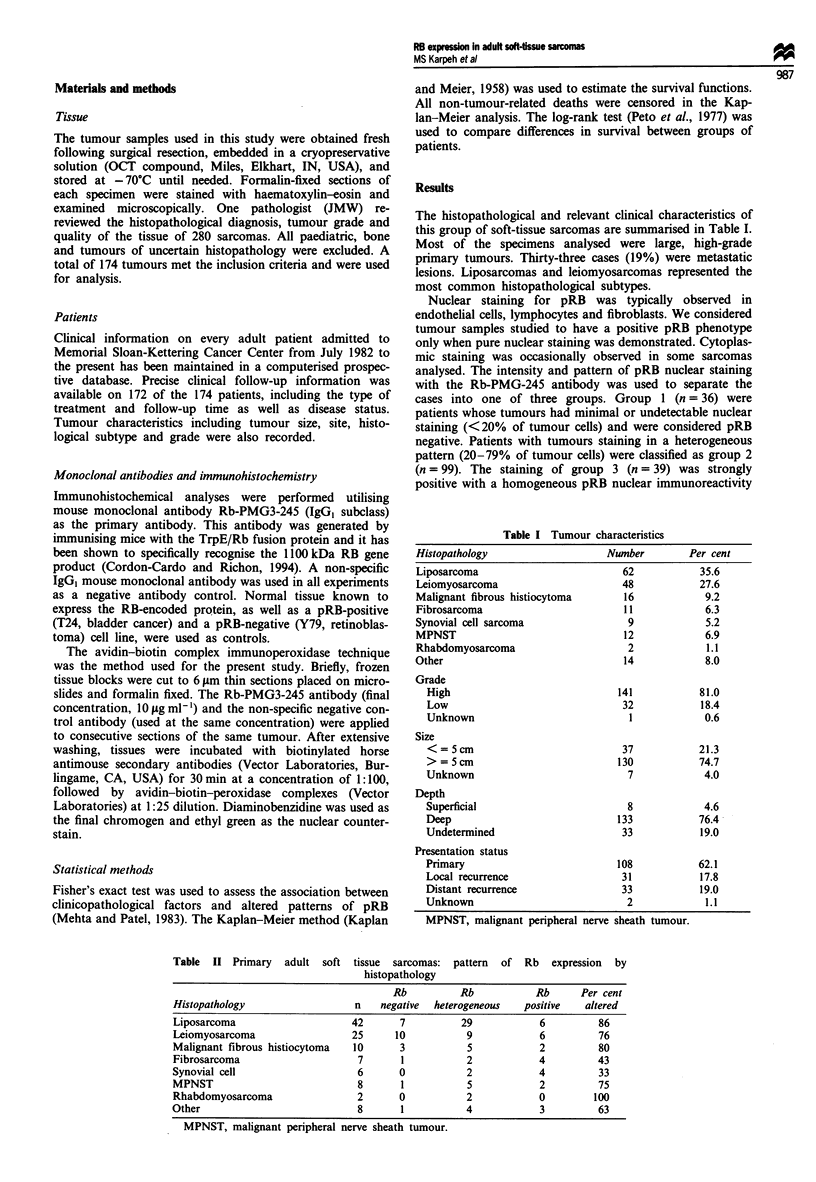
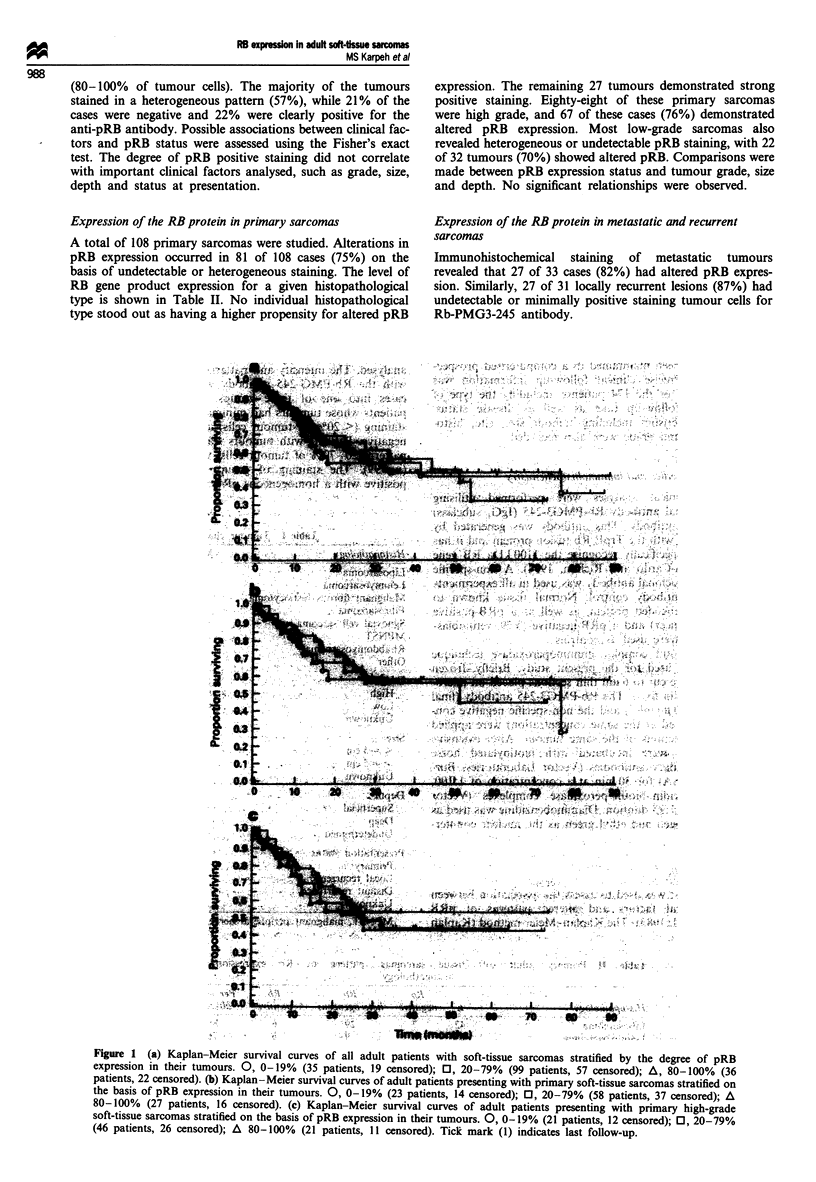
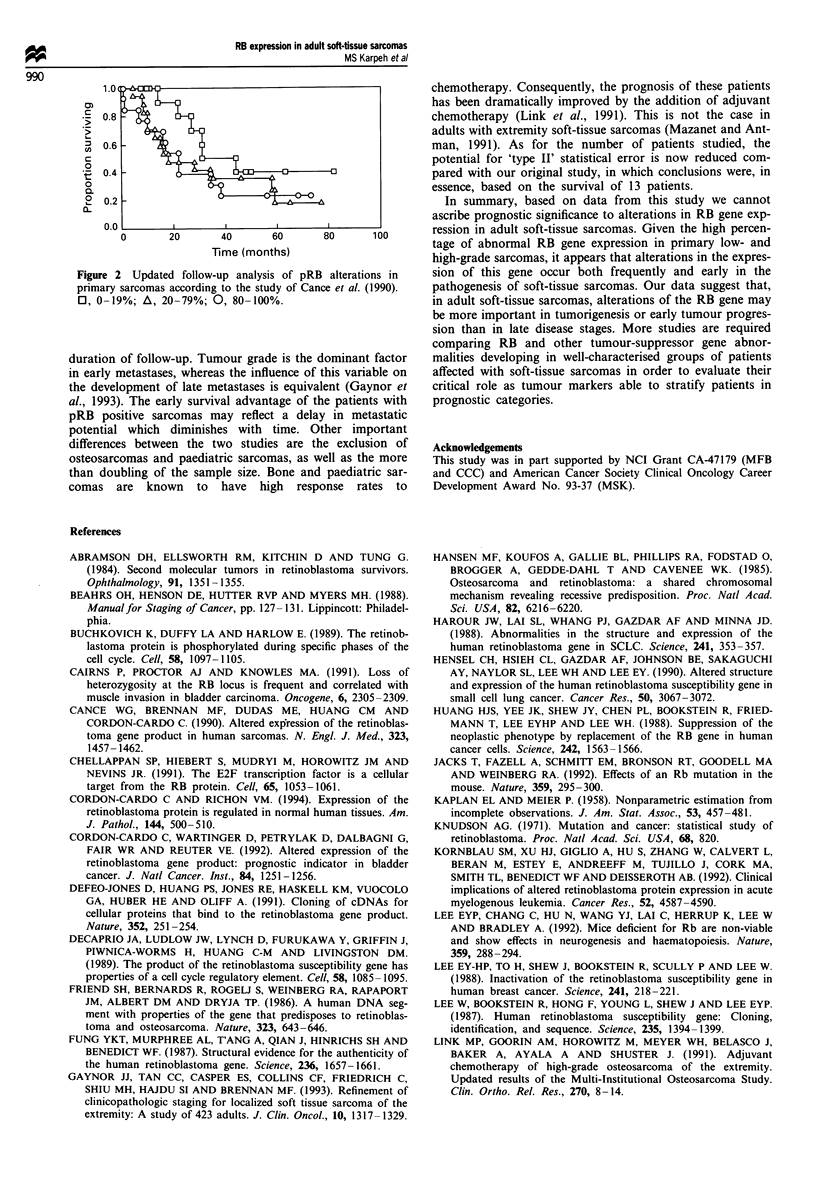
Altered expression of the retinoblastoma (RB) tumour-suppressor gene product (pRB) has been detected in sporadic bone and soft-tissue sarcomas. Earlier studies, analysing small cohorts of sarcoma patients, have suggested that these alterations are more commonly associated with high-grade tumours, metastatic lesions and poorer survival. This study was designed to re-examine the prevalence and clinical significance of altered pRB expression in a large and selected group of soft-tissue sarcomas from 174 adult patients. Representative tissue sections from these sarcomas were analysed by immunohistochemistry using a well-characterised anti-pRB monoclonal antibody. Tumours were considered to have a positive pRB phenotype only when pure nuclear staining was demonstrated, and cases were segregated into one of three groups. Group 1 (n = 36) were patients whose tumours have minimal or undetectable pRB nuclear staining (< 20% of tumour cells) and were considered pRB negative. Patients with tumours staining in a heterogeneous pattern (20-79% of tumour cells) were classified as group 2 (n = 99). The staining of group 3 (n = 39) was strongly positive with a homogeneous pRB nuclear immunoreactivity (80-100% of tumour cells). pRB alterations were frequently observed in both low- and high-grade lesions. Altered pRB expression did not correlate with known predictors of survival and was not itself an independent predictor of outcome in the long-term follow-up. These findings support earlier observations that alterations of pRB expression are common events in soft-tissue sarcomas; nevertheless, long-term follow-up results indicate that altered patterns of pRB expression do not influence clinical outcome of patients affected with soft-tissue sarcomas. It is postulated that RB alterations are primary events in human sarcomas and may be involved in tumorigenesis or early phases of tumour progression in these neoplasias.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Cairns P., Proctor A. J., Knowles M. A. Loss of heterozygosity at the RB locus is frequent and correlates with muscle invasion in bladder carcinoma. Oncogene. 1991 Dec;6(12):2305–2309. [PubMed] [Google Scholar]
- Cance W. G., Brennan M. F., Dudas M. E., Huang C. M., Cordon-Cardo C. Altered expression of the retinoblastoma gene product in human sarcomas. N Engl J Med. 1990 Nov 22;323(21):1457–1462. doi: 10.1056/NEJM199011223232105. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Richon V. M. Expression of the retinoblastoma protein is regulated in normal human tissues. Am J Pathol. 1994 Mar;144(3):500–510. [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C., Wartinger D., Petrylak D., Dalbagni G., Fair W. R., Fuks Z., Reuter V. E. Altered expression of the retinoblastoma gene product: prognostic indicator in bladder cancer. J Natl Cancer Inst. 1992 Aug 19;84(16):1251–1256. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Defeo-Jones D., Huang P. S., Jones R. E., Haskell K. M., Vuocolo G. A., Hanobik M. G., Huber H. E., Oliff A. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature. 1991 Jul 18;352(6332):251–254. doi: 10.1038/352251a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gaynor J. J., Tan C. C., Casper E. S., Collin C. F., Friedrich C., Shiu M., Hajdu S. I., Brennan M. F. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: a study of 423 adults. J Clin Oncol. 1992 Aug;10(8):1317–1329. doi: 10.1200/JCO.1992.10.8.1317. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel C. H., Hsieh C. L., Gazdar A. F., Johnson B. E., Sakaguchi A. Y., Naylor S. L., Lee W. H., Lee E. Y. Altered structure and expression of the human retinoblastoma susceptibility gene in small cell lung cancer. Cancer Res. 1990 May 15;50(10):3067–3072. [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblau S. M., Xu H. J., del Giglio A., Hu S. X., Zhang W., Calvert L., Beran M., Estey E., Andreeff M., Trujillo J. Clinical implications of decreased retinoblastoma protein expression in acute myelogenous leukemia. Cancer Res. 1992 Sep 1;52(17):4587–4590. [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Link M. P., Goorin A. M., Horowitz M., Meyer W. H., Belasco J., Baker A., Ayala A., Shuster J. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991 Sep;(270):8–14. [PubMed] [Google Scholar]
- Mazanet R., Antman K. H. Adjuvant therapy for sarcomas. Semin Oncol. 1991 Dec;18(6):603–612. [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann P. T., Simon M. A., Lee W. H., Slamon D. J. Studies of the retinoblastoma gene in human sarcomas. Oncogene. 1989 Jul;4(7):839–843. [PubMed] [Google Scholar]
- Russell W. O., Cohen J., Enzinger F., Hajdu S. I., Heise H., Martin R. G., Meissner W., Miller W. T., Schmitz R. L., Suit H. D. A clinical and pathological staging system for soft tissue sarcomas. Cancer. 1977 Oct;40(4):1562–1570. doi: 10.1002/1097-0142(197710)40:4<1562::aid-cncr2820400428>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Stratton M. R., Moss S., Warren W., Patterson H., Clark J., Fisher C., Fletcher C. D., Ball A., Thomas M., Gusterson B. A. Mutation of the p53 gene in human soft tissue sarcomas: association with abnormalities of the RB1 gene. Oncogene. 1990 Sep;5(9):1297–1301. [PubMed] [Google Scholar]
- Sumegi J., Uzvolgyi E., Klein G. Expression of the RB gene under the control of MuLV-LTR suppresses tumorigenicity of WERI-Rb-27 retinoblastoma cells in immunodefective mice. Cell Growth Differ. 1990 May;1(5):247–250. [PubMed] [Google Scholar]
- Szekely L., Jiang W. Q., Bulic-Jakus F., Rosen A., Ringertz N., Klein G., Wiman K. G. Cell type and differentiation dependent heterogeneity in retinoblastoma protein expression in SCID mouse fetuses. Cell Growth Differ. 1992 Mar;3(3):149–156. [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]
- Wunder J. S., Czitrom A. A., Kandel R., Andrulis I. L. Analysis of alterations in the retinoblastoma gene and tumor grade in bone and soft-tissue sarcomas. J Natl Cancer Inst. 1991 Feb 6;83(3):194–200. doi: 10.1093/jnci/83.3.194. [DOI] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Cagle P. T., Moore G. E., Benedict W. F. Absence of retinoblastoma protein expression in primary non-small cell lung carcinomas. Cancer Res. 1991 May 15;51(10):2735–2739. [PubMed] [Google Scholar]


