Abstract
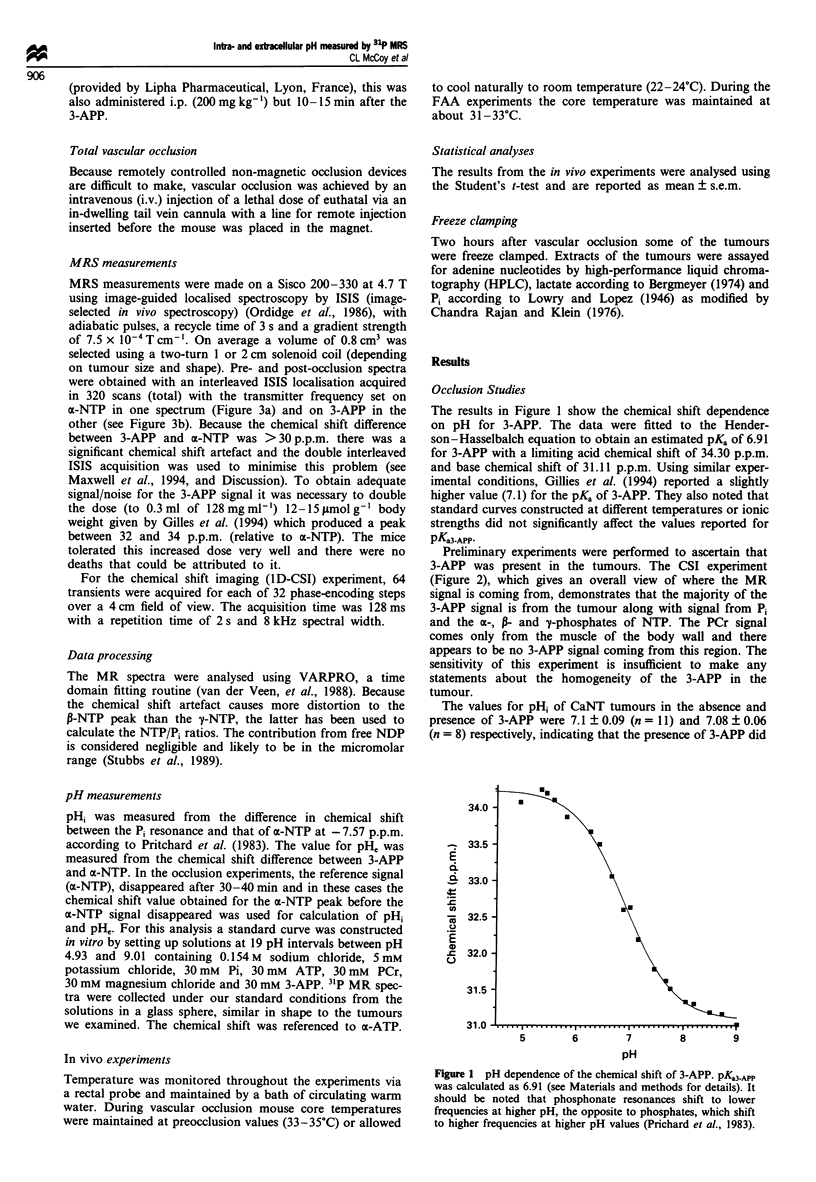
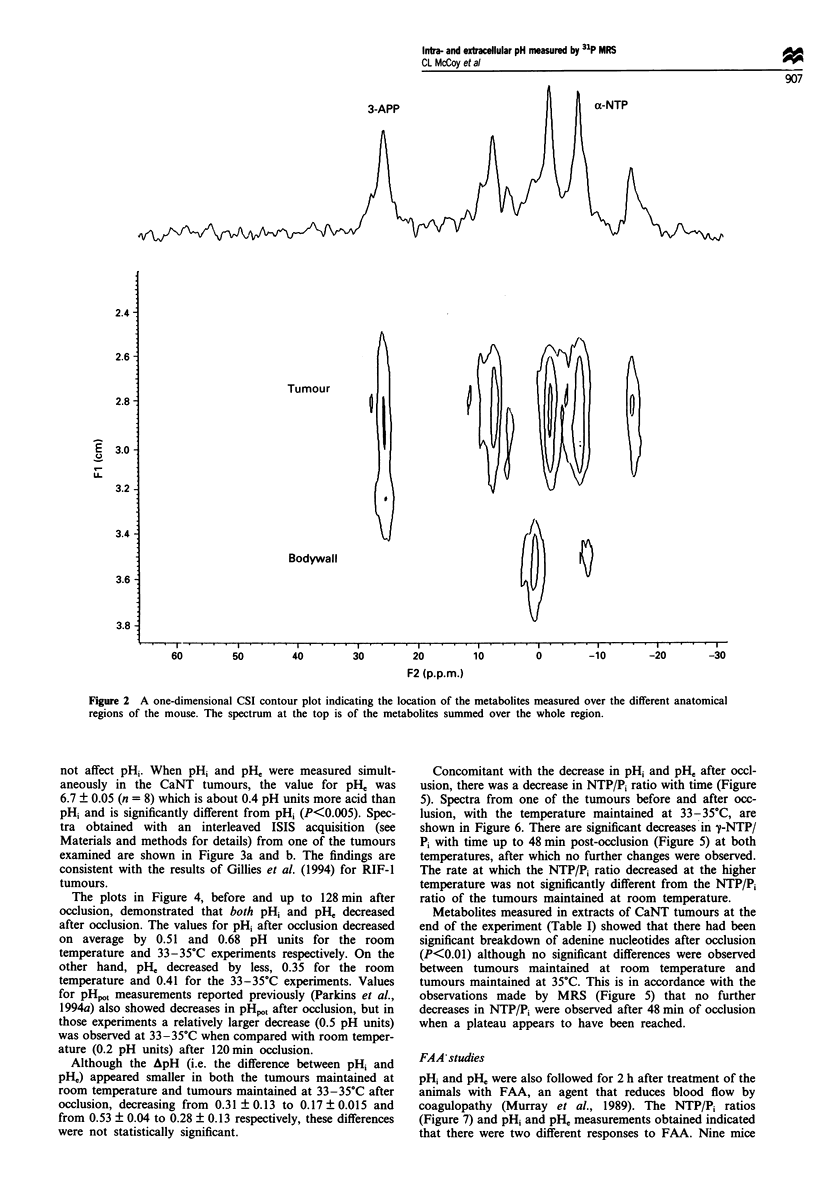
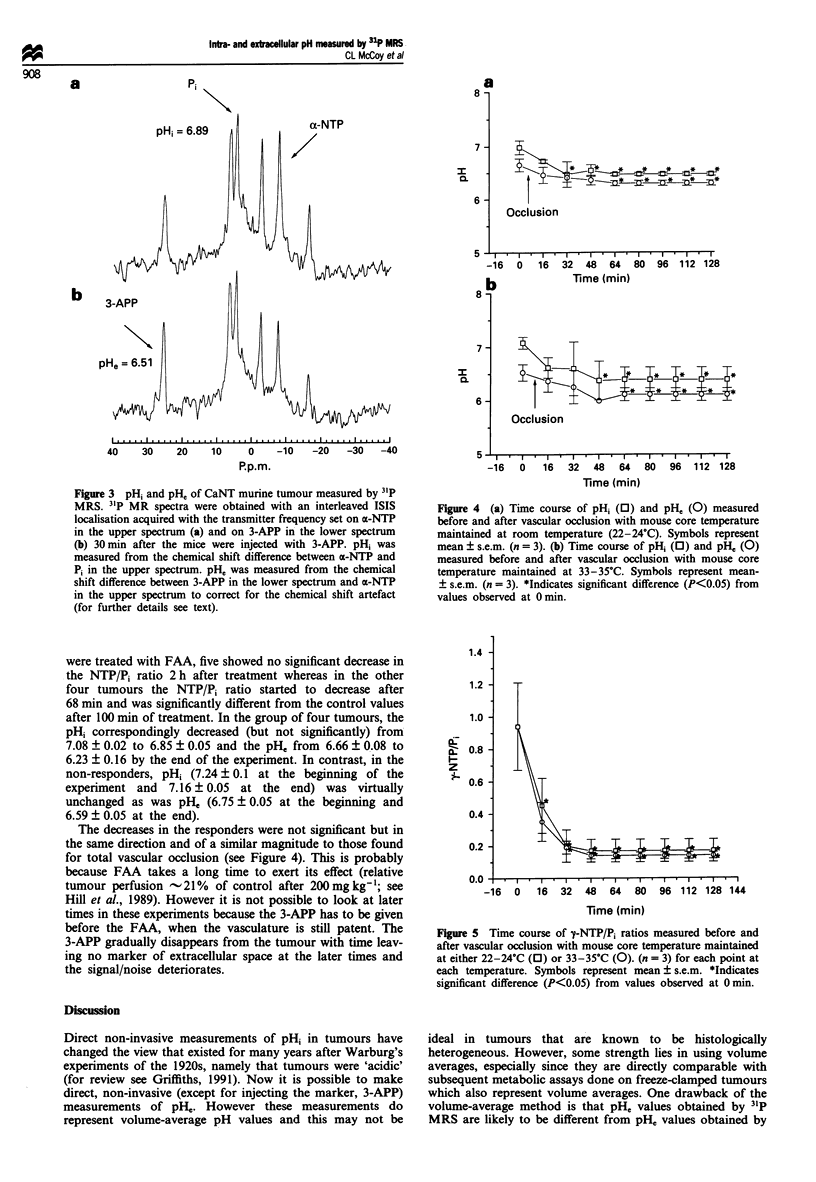
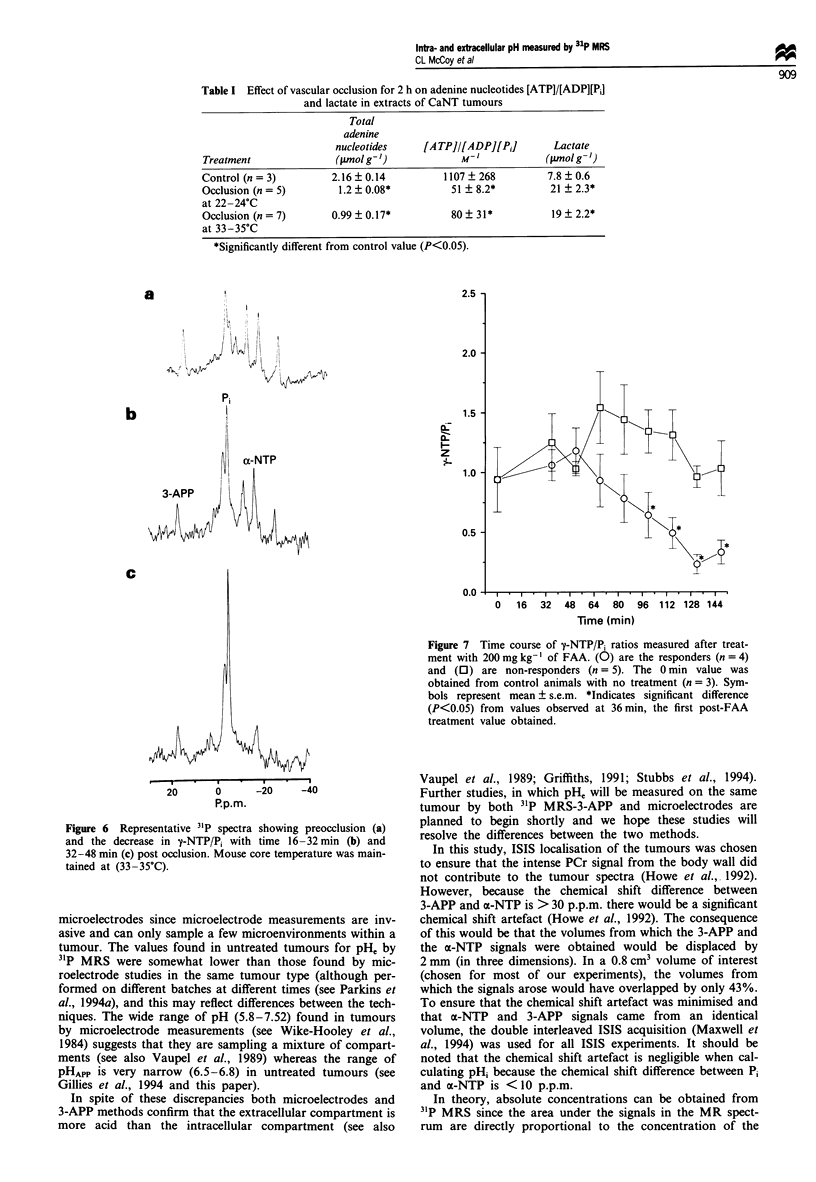
Intra- and extracellular pH (pHi and pHe) were measured simultaneously by 31P magnetic resonance spectroscopy (MRS) in CaNT tumours before and after blood flow modification. Before modification, pHi was 7.1 +/- 0.09 (n = 11) and pHe [measured with an MRS-visible extracellular marker, 3-aminopropyl phosphonate (3-APP)] was 6.7 +/- 0.05 (n = 8). Chemical shift imaging and localised MRS experiments showed that the 3-APP signal was only from the tumour, not surrounding tissue. After modification by vascular occlusion, independent of whether tumours were maintained at room temperature (22-24 degrees C) or kept warm (33-35 degrees C), there was a decrease in pHi and pHe with pHi decreasing to a greater extent. Qualitatively similar results were found using flavone acetic acid (FAA) as a blood flow modifier; only four out of nine tumours responded to FAA. Concomitant with the reduction of the pH gradient after modification was a decrease in the phosphorylation state of the adenine nucleotides measured either as ATP/Pi by MRS or [ATP]/[ADP][P(i)] in tumour extracts. These results indicate that the intracellular uptake of chemotherapeutic drugs which are dependent on the transmembrane pH gradient will not be enhanced in cells made ischaemic as a result of vascular shutdown.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ChandraRajan J., Klein L. Determination of inorganic phosphorus in the presence of organic phosphorus and high concentrations of proteins. Anal Biochem. 1976 May 7;72:407–412. doi: 10.1016/0003-2697(76)90548-0. [DOI] [PubMed] [Google Scholar]
- Evelhoch J. L., Bissery M. C., Chabot G. G., Simpson N. E., McCoy C. L., Heilbrun L. K., Corbett T. H. Flavone acetic acid (NSC 347512)-induced modulation of murine tumor physiology monitored by in vivo nuclear magnetic resonance spectroscopy. Cancer Res. 1988 Sep 1;48(17):4749–4755. [PubMed] [Google Scholar]
- Evelhoch J. L., Sapareto S. A., Jick D. E., Ackerman J. J. In vivo metabolic effects of hyperglycemia in murine radiation-induced fibrosarcoma: a 31P NMR investigation. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6496–6500. doi: 10.1073/pnas.81.20.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerweck L. E., Rhee J. G., Koutcher J. A., Song C. W., Urano M. Regulation of pH in murine tumor and muscle. Radiat Res. 1991 May;126(2):206–209. [PubMed] [Google Scholar]
- Gillies R. J., Liu Z., Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994 Jul;267(1 Pt 1):C195–C203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- Griffiths J. R. Are cancer cells acidic? Br J Cancer. 1991 Sep;64(3):425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S., Williams K. B., Denekamp J. Vascular collapse after flavone acetic acid: a possible mechanism of its anti-tumour action. Eur J Cancer Clin Oncol. 1989 Oct;25(10):1419–1424. doi: 10.1016/0277-5379(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Hahn G. M. Comparison between tumor pH and cell sensitivity to heat in RIF-1 tumors. Cancer Res. 1989 Jul 15;49(14):3734–3736. [PubMed] [Google Scholar]
- Howe F. A., Stubbs M., Rodrigues L. M., Griffiths J. R. An assessment of artefacts in localized and non-localized 31P MRS studies of phosphate metabolites and pH in rat tumours. NMR Biomed. 1993 Jan-Feb;6(1):43–52. doi: 10.1002/nbm.1940060108. [DOI] [PubMed] [Google Scholar]
- Hwang Y. C., Kim S. G., Evelhoch J. L., Seyedsadr M., Ackerman J. J. Modulation of murine radiation-induced fibrosarcoma-1 tumor metabolism and blood flow in situ via glucose and mannitol administration monitored by 31P and 2H nuclear magnetic resonance spectroscopy. Cancer Res. 1991 Jun 15;51(12):3108–3118. [PubMed] [Google Scholar]
- Jähde E., Volk T., Atema A., Smets L. A., Glüsenkamp K. H., Rajewsky M. F. pH in human tumor xenografts and transplanted rat tumors: effect of insulin, inorganic phosphate, and m-iodobenzylguanidine. Cancer Res. 1992 Nov 15;52(22):6209–6215. [PubMed] [Google Scholar]
- Maidorn R. P., Cragoe E. J., Jr, Tannock I. F. Therapeutic potential of analogues of amiloride: inhibition of the regulation of intracellular pH as a possible mechanism of tumour selective therapy. Br J Cancer. 1993 Feb;67(2):297–303. doi: 10.1038/bjc.1993.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. C., Smith K. A., Thurston G. Flavone acetic acid induces a coagulopathy in mice. Br J Cancer. 1989 Nov;60(5):729–733. doi: 10.1038/bjc.1989.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negendank W. Studies of human tumors by MRS: a review. NMR Biomed. 1992 Sep-Oct;5(5):303–324. doi: 10.1002/nbm.1940050518. [DOI] [PubMed] [Google Scholar]
- Newell K., Wood P., Stratford I., Tannock I. Effects of agents which inhibit the regulation of intracellular pH on murine solid tumours. Br J Cancer. 1992 Aug;66(2):311–317. doi: 10.1038/bjc.1992.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins C. S., Chadwick J. A., Chaplin D. J. Enhancement of chlorambucil cytotoxicity by combination with flavone acetic acid in a murine tumour. Anticancer Res. 1994 Jul-Aug;14(4A):1603–1608. [PubMed] [Google Scholar]
- Parkins C. S., Denekamp J., Chaplin D. J. Enhancement of mitomycin-C cytotoxicity by combination with flavone acetic acid in a murine tumour. Anticancer Res. 1993 Sep-Oct;13(5A):1437–1442. [PubMed] [Google Scholar]
- Parkins C. S., Hill S. A., Lonergan S. J., Horsman M. R., Chadwick J. A., Chaplin D. J. Ischaemia induced cell death in tumors: importance of temperature and pH. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):499–503. doi: 10.1016/0360-3016(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Prichard J. W., Alger J. R., Behar K. L., Petroff O. A., Shulman R. G. Cerebral metabolic studies in vivo by 31P NMR. Proc Natl Acad Sci U S A. 1983 May;80(9):2748–2751. doi: 10.1073/pnas.80.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Bhujwalla Z. M., Tozer G. M., Rodrigues L. M., Maxwell R. J., Morgan R., Howe F. A., Griffiths J. R. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992 Nov-Dec;5(6):351–359. doi: 10.1002/nbm.1940050606. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L. M., Griffiths J. R. Growth studies of subcutaneous rat tumours: comparison of 31P-NMR spectroscopy, acid extracts and histology. Br J Cancer. 1989 Nov;60(5):701–707. doi: 10.1038/bjc.1989.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L., Howe F. A., Wang J., Jeong K. S., Veech R. L., Griffiths J. R. Metabolic consequences of a reversed pH gradient in rat tumors. Cancer Res. 1994 Aug 1;54(15):4011–4016. [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Voorhees W. D., 3rd, Babbs C. F. Hydralazine-enhanced selective heating of transmissible venereal tumor implants in dogs. Eur J Cancer Clin Oncol. 1982 Oct;18(10):1027–1033. doi: 10.1016/0277-5379(82)90252-8. [DOI] [PubMed] [Google Scholar]
- Weber G., Stubbs M., Morris H. P. Metabolism of hepatomas of different growth rates in situ and during ischemia. Cancer Res. 1971 Dec;31(12):2177–2183. [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Krebs H. A., Stubbs M., Page M. A., Morris H. P., Weber G. Metabolism of renal tumors in situ and during ischemia. Cancer Res. 1970 Jul;30(7):2049–2054. [PubMed] [Google Scholar]
- van der Veen J. W., de Beer R., Luyten P. R., van Ormondt D. Accurate quantification of in vivo 31P NMR signals using the variable projection method and prior knowledge. Magn Reson Med. 1988 Jan;6(1):92–98. doi: 10.1002/mrm.1910060111. [DOI] [PubMed] [Google Scholar]


