Abstract
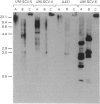
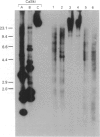
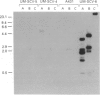
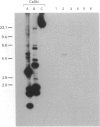
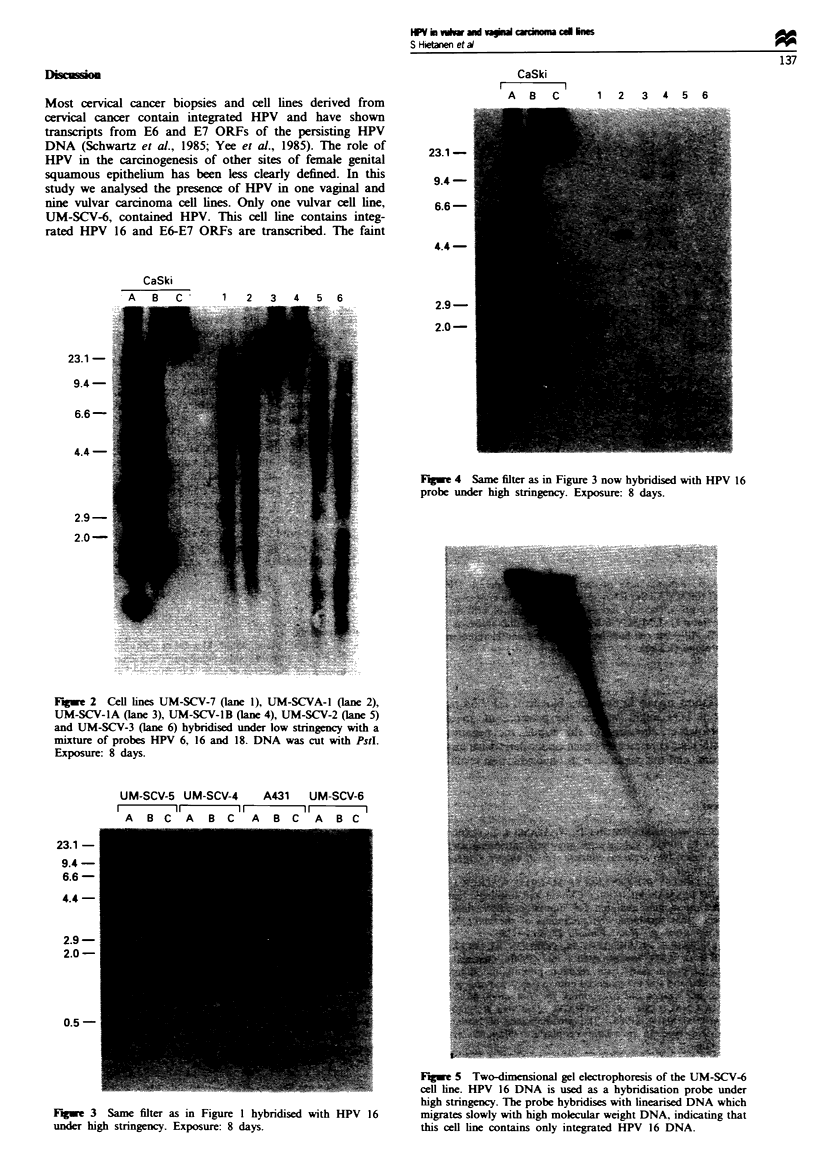
A number of reports associate human papillomavirus (HPV) with cervical cancer and cancer cell lines derived from this tumour type. Considerably fewer reports have focused on the role of HPV in carcinomas from other sites of female anogenital squamous epithelia. In this study we have tested for the presence of HPV in eight low-passage vulvar carcinoma cell lines and one extensively passaged cell line, A431. One cell line from a primary vaginal carcinoma was included. The presence of the HPV was evaluated by the polymerase chain reaction (PCR), by Southern blot analysis and by two-dimensional gel electrophoresis. General primer-mediated PCR was applied by using primers from the L1 region, E1 region and HPV 16 E7 region. Southern blot hybridisation was performed under low-stringency conditions (Tm = -35 degrees C) using a whole genomic HPV 6/16/18 probe mixture and under high stringency conditions (Tm = -18 degrees C) with the whole genomic probes of HPV 16 and 33. HPV 16 E6-E7 mRNA was assessed by ribonuclease protection assay (RPA). HPV was found in only one vulvar carcinoma cell line, UM-SCV-6. The identified type, HPV 16, was integrated in the cell genome and could be amplified with all primers used. Also E6-E7 transcripts were found in these cells. Five original tumour biopsies were available from the HPV-negative cell lines for in situ hybridisation. All these were HPV negative with both the HPV 6/16/18 screening probe mixture under low stringency and the HPV 16 probe under high stringency. The results indicate that vulvar carcinoma cell lines contain HPV less frequently than cervical carcinoma cell lines and suggest that a significant proportion of vulvar carcinomas may evolve by an HPV-independent mechanism.
Full text
PDF
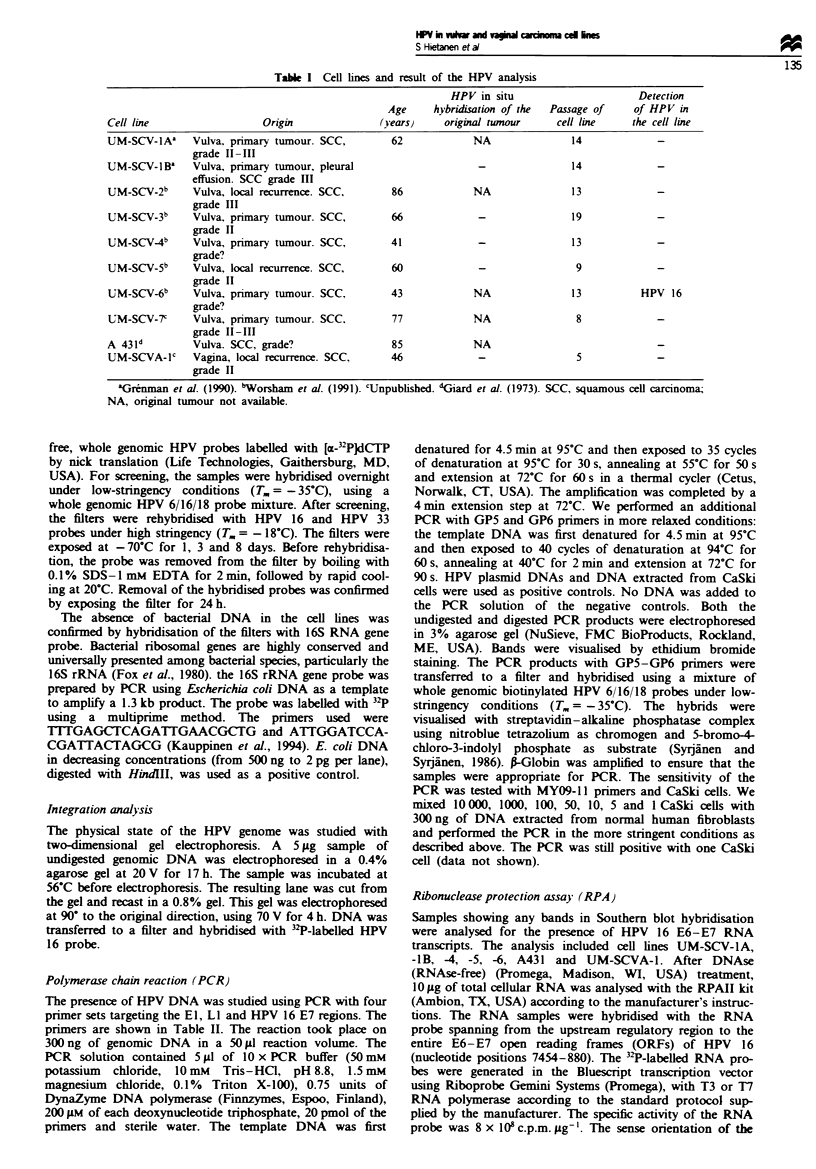
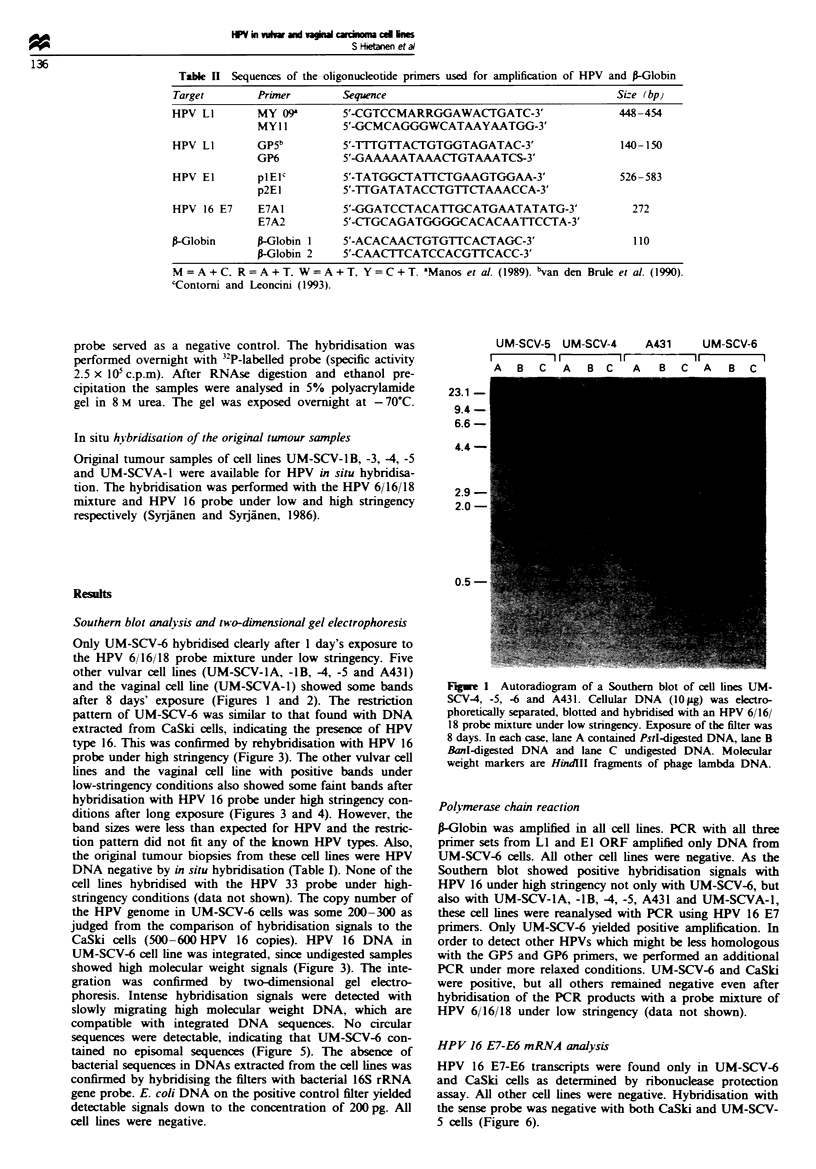


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen W. A., Franquemont D. W., Williams J., Taylor P. T., Crum C. P. Vulvar squamous cell carcinoma and papillomaviruses: two separate entities? Am J Obstet Gynecol. 1991 Aug;165(2):329–336. doi: 10.1016/0002-9378(91)90086-7. [DOI] [PubMed] [Google Scholar]
- Bloss J. D., Liao S. Y., Wilczynski S. P., Macri C., Walker J., Peake M., Berman M. L. Clinical and histologic features of vulvar carcinomas analyzed for human papillomavirus status: evidence that squamous cell carcinoma of the vulva has more than one etiology. Hum Pathol. 1991 Jul;22(7):711–718. doi: 10.1016/0046-8177(91)90294-y. [DOI] [PubMed] [Google Scholar]
- Buscema J., Naghashfar Z., Sawada E., Daniel R., Woodruff J. D., Shah K. The predominance of human papillomavirus type 16 in vulvar neoplasia. Obstet Gynecol. 1988 Apr;71(4):601–606. [PubMed] [Google Scholar]
- Contorni M., Leoncini P. Typing of human papillomavirus DNAs by restriction endonuclease mapping of the PCR products. J Virol Methods. 1993 Jan;41(1):29–36. doi: 10.1016/0166-0934(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Grénman S. E., Van Dyke D. L., Worsham M. J., England B., McClatchey K. D., Hopkins M., Babu V. R., Grénman R., Carey T. E. Phenotypic characterization, karyotype analysis and in vitro tamoxifen sensitivity of new ER-negative vulvar carcinoma cell lines, UM-SCV-1A and UM-SCV-1B. Int J Cancer. 1990 May 15;45(5):920–927. doi: 10.1002/ijc.2910450524. [DOI] [PubMed] [Google Scholar]
- Ikenberg H., Runge M., Göppinger A., Pfleiderer A. Human papillomavirus DNA in invasive carcinoma of the vagina. Obstet Gynecol. 1990 Sep;76(3 Pt 1):432–438. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Jones R. W., McLean M. R., Currie J. L., Woodruff J. D., Shah K. V., Kurman R. J. Possible etiologic heterogeneity of vulvar intraepithelial neoplasia. A correlation of pathologic characteristics with human papillomavirus detection by in situ hybridization and polymerase chain reaction. Cancer. 1991 Mar 15;67(6):1599–1607. doi: 10.1002/1097-0142(19910315)67:6<1599::aid-cncr2820670622>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Planner R. S., Hobbs J. B. Intraepithelial and invasive neoplasia of the vulva in association with human papillomavirus infection. J Reprod Med. 1988 Jun;33(6):503–509. [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Sherman K. J., Daling J. R., Chu J., Weiss N. S., Ashley R. L., Corey L. Genital warts, other sexually transmitted diseases, and vulvar cancer. Epidemiology. 1991 Jul;2(4):257–262. doi: 10.1097/00001648-199107000-00004. [DOI] [PubMed] [Google Scholar]
- Syrjänen K., Mäntyjärvi R., Saarikoski S., Väyrynen M., Syrjänen S., Parkkinen S., Yliskoski M., Saastamoinen J., Castren O. Factors associated with progression of cervical human papillomavirus (HPV) infections into carcinoma in situ during a long-term prospective follow-up. Br J Obstet Gynaecol. 1988 Nov;95(11):1096–1102. doi: 10.1111/j.1471-0528.1988.tb06785.x. [DOI] [PubMed] [Google Scholar]
- Syrjänen S., Syrjänen K. An improved in situ DNA hybridization protocol for detection of human papillomavirus (HPV) DNA sequences in paraffin-embedded biopsies. J Virol Methods. 1986 Nov;14(3-4):293–304. doi: 10.1016/0166-0934(86)90031-5. [DOI] [PubMed] [Google Scholar]
- Toki T., Kurman R. J., Park J. S., Kessis T., Daniel R. W., Shah K. V. Probable nonpapillomavirus etiology of squamous cell carcinoma of the vulva in older women: a clinicopathologic study using in situ hybridization and polymerase chain reaction. Int J Gynecol Pathol. 1991;10(2):107–125. doi: 10.1097/00004347-199104000-00001. [DOI] [PubMed] [Google Scholar]
- Worsham M. J., Van Dyke D. L., Grenman S. E., Grenman R., Hopkins M. P., Roberts J. A., Gasser K. M., Schwartz D. R., Carey T. E. Consistent chromosome abnormalities in squamous cell carcinoma of the vulva. Genes Chromosomes Cancer. 1991 Nov;3(6):420–432. doi: 10.1002/gcc.2870030604. [DOI] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- van den Brule A. J., Snijders P. J., Gordijn R. L., Bleker O. P., Meijer C. J., Walboomers J. M. General primer-mediated polymerase chain reaction permits the detection of sequenced and still unsequenced human papillomavirus genotypes in cervical scrapes and carcinomas. Int J Cancer. 1990 Apr 15;45(4):644–649. doi: 10.1002/ijc.2910450412. [DOI] [PubMed] [Google Scholar]