Figure 3. Experimental methods to determine the mechanism of multisite phosphorylation.
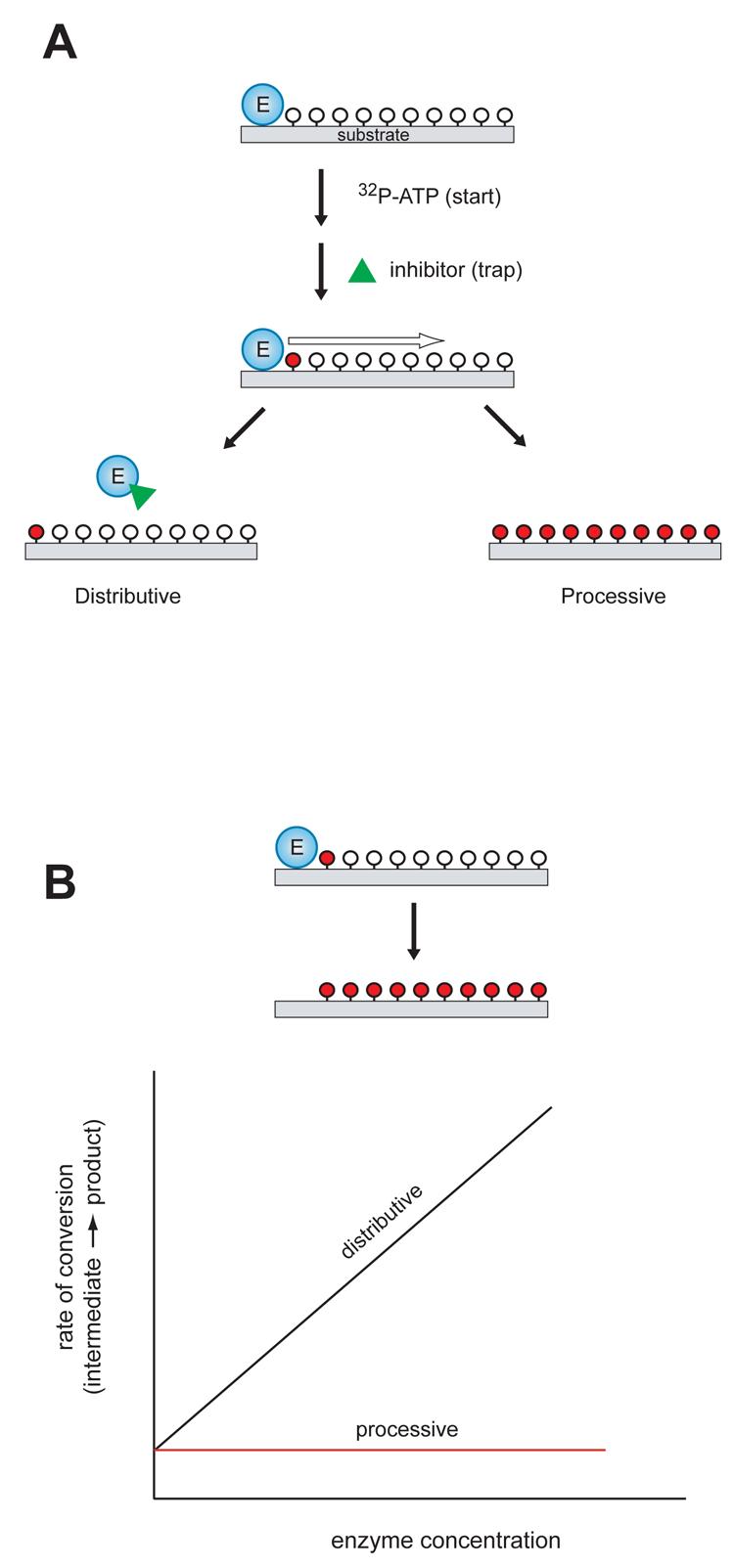
A, In the “start-trap” strategy, ATP is added to the enzyme-substrate complex together with an inhibitor that can trap the free enzyme (green triangle). If the enzyme phosphorylates the substrate by a non-processive mechanism, the inhibitor traps the free enzyme after dissociation from the substrate, stopping the reaction after one round of phosphorylation. In a processive mechanism, the inhibitor does not influence the rate or extent of substrate phosphorylation. Unphosphorylated sites are shown indicated by open circles; phosphorylated sites are indicated by red circles. B, Kinetic experiments with varying enzyme concentration. For processive phosphorylation, the enzyme and substrate remain bound during the multiple phosphorylation events, so the rate of progression from an intermediate phosphorylated form to fully phosphorylated product is independent of the enzyme concentration (red line). For a non-processive mechanism of phosphorylation, the rate of progression is dependent on enzyme concentration (black line). Unphosphorylated sites are shown indicated by open circles; phosphorylated sites are indicated by red circles.
